doc
... in many of the still unexplored regions of the electromagnetic spectrum. As a laser 1, it produces light in a single wavelength. Ordinary white light contains particles of light, or photons, with a broad range of different colors. So, when white light strikes an object, it causes a multiplicity of r ...
... in many of the still unexplored regions of the electromagnetic spectrum. As a laser 1, it produces light in a single wavelength. Ordinary white light contains particles of light, or photons, with a broad range of different colors. So, when white light strikes an object, it causes a multiplicity of r ...
Introduction, Introduction to lasers, Properties of light
... if the photon is absorbed – neither of the detectors clicks if the photon takes the other path – at the last bs it has an equal chance to be transmitted or reflected – the two detectors click with equal frequencies - the interference has been destroyed by the presence of the absorber the presence of ...
... if the photon is absorbed – neither of the detectors clicks if the photon takes the other path – at the last bs it has an equal chance to be transmitted or reflected – the two detectors click with equal frequencies - the interference has been destroyed by the presence of the absorber the presence of ...
Definitions are in Book
... 1) Why are the noble gases used for condensed electron configurations? There is due to the fact that this atoms are extremely stable. It takes a fair amount of energy to change their electron configurations. You’ll notice that it takes a lot of energy to remove an electron and even takes energy to a ...
... 1) Why are the noble gases used for condensed electron configurations? There is due to the fact that this atoms are extremely stable. It takes a fair amount of energy to change their electron configurations. You’ll notice that it takes a lot of energy to remove an electron and even takes energy to a ...
Chem vocab quiz definitons
... Condensation is the change of state from gas to liquid. Sublimation the change of state from solid directly to gas. Solubility is a measure of how well a substance can dissolve in another substance at a given temperature. Solute is the part of the solution that is dissolved by another substance. Sol ...
... Condensation is the change of state from gas to liquid. Sublimation the change of state from solid directly to gas. Solubility is a measure of how well a substance can dissolve in another substance at a given temperature. Solute is the part of the solution that is dissolved by another substance. Sol ...
Elements PPT
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
... the second can hold eight so it needs two more to be stable, that means that oxygen wants to combine with other elements or itself. ...
ELECTRONS IN ATOMS
... It is called a quantum. 4. Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. move an electron from its present energy level to the next lower one b. maintain an electron in its present energy level c. move an electron from ...
... It is called a quantum. 4. Circle the letter of the term that completes the sentence correctly. A quantum of energy is the amount of energy required to a. move an electron from its present energy level to the next lower one b. maintain an electron in its present energy level c. move an electron from ...
Exam 1 Topics to Review (McMurry Chpts 1
... f. Know the number of orbitals in each type of subshell (s, p, d, f subshells), and that a maximum of two electrons can be in each orbital. 7. Electron configurations and orbital diagrams: g. Know how to write both full and condensed electron configurations for atoms and ions of main group ele ...
... f. Know the number of orbitals in each type of subshell (s, p, d, f subshells), and that a maximum of two electrons can be in each orbital. 7. Electron configurations and orbital diagrams: g. Know how to write both full and condensed electron configurations for atoms and ions of main group ele ...
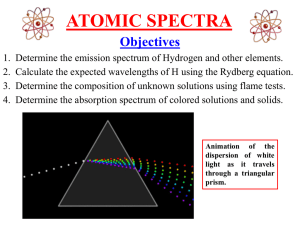
Atomic_spectra
... 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. ...
... 1861, experiments by Kirchhoff and Bunsen demonstrated that each element, when heated to incandescence, gave off a characteristic color of light. When the light was separated into its constituent wavelengths by a prism, each element displayed a unique pattern or emission spectrum. ...
Spectroscopy - Universität Wien
... EPR: Electron Paramagnetic Resonance (dt. Elektronenspinresonanz, ESR) → Probes energy differences between allowed spin states of electrons (whose energies are in the microwave range) → Principle similar to that of NMR: No external magnetic field: all spin states are equal (S = 1/2) With strong exte ...
... EPR: Electron Paramagnetic Resonance (dt. Elektronenspinresonanz, ESR) → Probes energy differences between allowed spin states of electrons (whose energies are in the microwave range) → Principle similar to that of NMR: No external magnetic field: all spin states are equal (S = 1/2) With strong exte ...
Degrees of freedom
... we say that the molecule has three degrees of freedom. It is therefore sensible to suppose that one-third of the total energy is associated with each degree of freedom, and this is known as Boltzmann’s law of equipartition of energy. Thus each degree of freedom has an amount of energy ½ RT associate ...
... we say that the molecule has three degrees of freedom. It is therefore sensible to suppose that one-third of the total energy is associated with each degree of freedom, and this is known as Boltzmann’s law of equipartition of energy. Thus each degree of freedom has an amount of energy ½ RT associate ...
CHEMISTRY – UNITS 3 and 4 REVIEW PACKET Name Date
... fission reaction is much greater than the energy released from a chemical reaction because in a fission reaction (1) mass is converted into energy (2) energy is converted into mass (3) ionic bonds are broken (4) covalent bonds are broken 5. How many days are required for 200. grams of radon-222 to d ...
... fission reaction is much greater than the energy released from a chemical reaction because in a fission reaction (1) mass is converted into energy (2) energy is converted into mass (3) ionic bonds are broken (4) covalent bonds are broken 5. How many days are required for 200. grams of radon-222 to d ...
The electron! Speed and energy notes
... •Niels Bohr in 1913 proposed a quantum model for the hydrogen atom which correctly predicted the frequencies of the lines (colors) in hydrogen’s atomic emissions spectrum. ...
... •Niels Bohr in 1913 proposed a quantum model for the hydrogen atom which correctly predicted the frequencies of the lines (colors) in hydrogen’s atomic emissions spectrum. ...
Chapter 11 Laser
... the ratio of the populations of the energy levels, will increase. Since the population of the normal state was so much larger than that of the excited state, an enormously intense beam of light would be required to increase the population of the excited states to a value comparable to or greater tha ...
... the ratio of the populations of the energy levels, will increase. Since the population of the normal state was so much larger than that of the excited state, an enormously intense beam of light would be required to increase the population of the excited states to a value comparable to or greater tha ...
Atomic Structure and Periodicity
... The closer an electron is to the nucleus, the more difficult it is to remove A sodium atom does not have as many valence electrons as a chlorine atom does. 3s electrons are lower in energy than 2p electrons Phosphorus and nitrogen are in the same row of the periodic table They each need one electron ...
... The closer an electron is to the nucleus, the more difficult it is to remove A sodium atom does not have as many valence electrons as a chlorine atom does. 3s electrons are lower in energy than 2p electrons Phosphorus and nitrogen are in the same row of the periodic table They each need one electron ...
final exam practice test - Clayton State University
... properties of gases and no others? I. At constant temperature, the pressure increases as the volume of a definite mass of a gas increases. II. At constant pressure, the volume of a definite mass of a gas increases as the temperature increases. III. At reasonable temperatures and pressures gases cons ...
... properties of gases and no others? I. At constant temperature, the pressure increases as the volume of a definite mass of a gas increases. II. At constant pressure, the volume of a definite mass of a gas increases as the temperature increases. III. At reasonable temperatures and pressures gases cons ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.