0321813545_07_final
... Know that electrons and photons behave in similar ways: both can act as particles and as waves. Know that photons and electrons, even when viewed as streams of particles, still display diffraction and interference patterns in a double‐slit experiment. Use de Broglie’s relation to interconvert wa ...
... Know that electrons and photons behave in similar ways: both can act as particles and as waves. Know that photons and electrons, even when viewed as streams of particles, still display diffraction and interference patterns in a double‐slit experiment. Use de Broglie’s relation to interconvert wa ...
Waves and Energy
... Note that because only certain atomic energies are possible, only certain frequencies of radiation can be emitted. You can compare hydrogen's seven atomic orbits to seven rungs on a ladder. A person can climb up or down the ladder only from rung to rung. Similarly, the hydrogen atom's electron ...
... Note that because only certain atomic energies are possible, only certain frequencies of radiation can be emitted. You can compare hydrogen's seven atomic orbits to seven rungs on a ladder. A person can climb up or down the ladder only from rung to rung. Similarly, the hydrogen atom's electron ...
NOTES: 2.1 - Intro to Chemistry
... ATOM: smallest unit of matter that retains the physical and chemical properties of its element ● three subatomic particles: ...
... ATOM: smallest unit of matter that retains the physical and chemical properties of its element ● three subatomic particles: ...
Physics Review
... -1 eV 1, 2, and 3 eV only -3 eV -5 eV 4, 5, and 9 eV only 1, 3, 5, and 10 eV only -10 eV 1, 5, 7, and 10 eV only -14 eV Since the original photons have a range of energies, one would expect a range of emitted photons with no particular energies. ...
... -1 eV 1, 2, and 3 eV only -3 eV -5 eV 4, 5, and 9 eV only 1, 3, 5, and 10 eV only -10 eV 1, 5, 7, and 10 eV only -14 eV Since the original photons have a range of energies, one would expect a range of emitted photons with no particular energies. ...
Lesson7
... • Some ‘pre-dissociation’ occurs in the B→X (SchumannRunge) system before the dissociation limit. This occurs because a ‘repulsive’ state crosses the B electronic state and a radiationless transition takes place. The repulsive state is unstable and dissociation takes place. Note that both atomic fra ...
... • Some ‘pre-dissociation’ occurs in the B→X (SchumannRunge) system before the dissociation limit. This occurs because a ‘repulsive’ state crosses the B electronic state and a radiationless transition takes place. The repulsive state is unstable and dissociation takes place. Note that both atomic fra ...
Modules to examine on the Arrangement of Electrons in Atoms website
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
Ch 5 - Electrons in Atoms
... If the light is not white • By electrifying a gas (like Neon) or exciting other elements with enough energy scientists can get it to give off colors. • Passing this light through a prism or diffraction grating does something different. ...
... If the light is not white • By electrifying a gas (like Neon) or exciting other elements with enough energy scientists can get it to give off colors. • Passing this light through a prism or diffraction grating does something different. ...
chapter 7: atomic structure and periodicity
... Energy is released (in the form of photons) when a e- moves from a _______________ to ______________________ orbit. 4) The energy of the photons emitted or absorbed is equal to the difference between the 2 orbit energies. This explains why only certain lines of specific wavelengths appear on a line ...
... Energy is released (in the form of photons) when a e- moves from a _______________ to ______________________ orbit. 4) The energy of the photons emitted or absorbed is equal to the difference between the 2 orbit energies. This explains why only certain lines of specific wavelengths appear on a line ...
LAMB SHIFT & VACUUM POLARIZATION CORRECTIONS TO THE
... charge distribution of the negative-energy electrons is different from that of the free-field case. In the electron-positron language this is due to the fact that a virtual electron-positron pair created in the Coulomb field behaves in such a way that the electron tends to be attracted to the nucleu ...
... charge distribution of the negative-energy electrons is different from that of the free-field case. In the electron-positron language this is due to the fact that a virtual electron-positron pair created in the Coulomb field behaves in such a way that the electron tends to be attracted to the nucleu ...
EM Notes
... optics, most long distance phone calls were sent via microwave. • Remote sensing; radars use microwaves to detect range and speed of remote objects. Used in doppler radar weather, air traffic control, navigation of ships, and of course, speed limit ...
... optics, most long distance phone calls were sent via microwave. • Remote sensing; radars use microwaves to detect range and speed of remote objects. Used in doppler radar weather, air traffic control, navigation of ships, and of course, speed limit ...
PHY215: Study Guide for Introductory Quantum Mechanics Explain 1. Cathode Ray tubes, Cathode rays, and the generation of X‐rays.
... a) Discovering the photoelectric effect. b) Demonstrating the wave nature of matter. c) Explaining light diffraction. d) Deriving Rydberg’s formula. e) Discovering the uncertainty principle. 2. The Copenhagen interpretation of Quantum Mechanics includes all of the items below except a) Complementari ...
... a) Discovering the photoelectric effect. b) Demonstrating the wave nature of matter. c) Explaining light diffraction. d) Deriving Rydberg’s formula. e) Discovering the uncertainty principle. 2. The Copenhagen interpretation of Quantum Mechanics includes all of the items below except a) Complementari ...
PE EFFECT - cranson
... A new theory of light: • Electromagnetic waves carry discrete energy packets • The energy per packet depends on wavelength, explaining Lenard’s threshold frequency. • More intense light corresponds to more photons, not higher energy photons. This was published in his famous 1905 paper: “On a Heurist ...
... A new theory of light: • Electromagnetic waves carry discrete energy packets • The energy per packet depends on wavelength, explaining Lenard’s threshold frequency. • More intense light corresponds to more photons, not higher energy photons. This was published in his famous 1905 paper: “On a Heurist ...
History of "s,p,d,f"
... discover, convenient to use. For single electrons we have: 1. For single-electron states the letter code s p d f g h ... is used in one-to-one correspondence with the values of the orbital angular momentum quantum number l : 0 1 2 3 4 5 .... For example, an electron with l = 2 is said to be a d elec ...
... discover, convenient to use. For single electrons we have: 1. For single-electron states the letter code s p d f g h ... is used in one-to-one correspondence with the values of the orbital angular momentum quantum number l : 0 1 2 3 4 5 .... For example, an electron with l = 2 is said to be a d elec ...
Chapter 2
... ion - any atom with a positive or negative charge anion - atom with a NEGATIVE charge cation - atom with a POSITIVE charge ...
... ion - any atom with a positive or negative charge anion - atom with a NEGATIVE charge cation - atom with a POSITIVE charge ...
The Atomic Emission Spectra of Hydrogen, Deuterium
... à Sample. Repeat for the deuterium discharge tube and the large (bright!) sodium source lamp. ...
... à Sample. Repeat for the deuterium discharge tube and the large (bright!) sodium source lamp. ...
Homework Handout #3
... 18.1 T are in us. Here the Larmor frequency for a proton is 800 MHz) So the vernacular is that an instrument with an 18.1 T magnet is called an 800MHz NMR. ...
... 18.1 T are in us. Here the Larmor frequency for a proton is 800 MHz) So the vernacular is that an instrument with an 18.1 T magnet is called an 800MHz NMR. ...
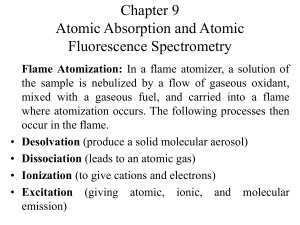
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cu ...
... sample is atomized in a short period, and the average residence time of the atoms in the optical path is a second or more. A few microliters of sample are first evaporated at a low temperature and then ashed at a somewhat higher temperature in an electrically heated graphite tube or in a graphite cu ...
PPT - kimscience.com
... atomic theory 1890’s X-rays given off from anode when cathode operating (light energy) ...
... atomic theory 1890’s X-rays given off from anode when cathode operating (light energy) ...
No Slide Title
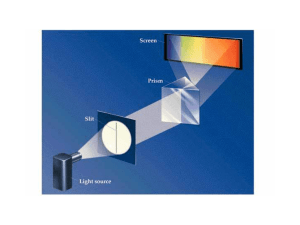
... passing from one substance to another) Dispersion: White light can be separated into colors. Diffraction: Light sources interact to give both constructive and destructive interference. ...
... passing from one substance to another) Dispersion: White light can be separated into colors. Diffraction: Light sources interact to give both constructive and destructive interference. ...
X-ray fluorescence

X-ray fluorescence (XRF) is the emission of characteristic ""secondary"" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. The phenomenon is widely used for elemental analysis and chemical analysis, particularly in the investigation of metals, glass, ceramics and building materials, and for research in geochemistry, forensic science and archaeology.