Spectroscopy
... The constant R is called the Rydberg constant. Planck’s constant is h; the speed of light is c. In the Bohr Model, the Rydberg constant is predicted to be R 1.0975 x10 7 m 1 . We shall determine R experimentally by observing the emission spectrum of Hydrogen. The four bright spectral lines in the ...
... The constant R is called the Rydberg constant. Planck’s constant is h; the speed of light is c. In the Bohr Model, the Rydberg constant is predicted to be R 1.0975 x10 7 m 1 . We shall determine R experimentally by observing the emission spectrum of Hydrogen. The four bright spectral lines in the ...
12 Using LEDs to Measure Planck`s Constant
... scales that were used had units of nanometers (nm). One nanometer equals 1 x 10-9 meters. This unit is frequently used to describe the wavelengths of visible light. The wavelength of light is just another way to indicate the energy (and color) of a photon that is emitted. In our investigations with ...
... scales that were used had units of nanometers (nm). One nanometer equals 1 x 10-9 meters. This unit is frequently used to describe the wavelengths of visible light. The wavelength of light is just another way to indicate the energy (and color) of a photon that is emitted. In our investigations with ...
The Helium Atom
... The Helium Atom The Helium Atom poses a problem that (like all other atomic and molecular systems) can only be solved through approximations – there exists no closed analytical solution. A spherical (“central-force”) problem similar to the hydrogen atom There exist two electrons and one nucleu ...
... The Helium Atom The Helium Atom poses a problem that (like all other atomic and molecular systems) can only be solved through approximations – there exists no closed analytical solution. A spherical (“central-force”) problem similar to the hydrogen atom There exist two electrons and one nucleu ...
Chapter 40
... Einstein extended Planck’s concept of quantization to electromagnetic waves. All electromagnetic radiation of frequency ƒ from any source can be considered a stream of quanta, now called photons. Each photon has an energy E and moves at the speed of light in a vacuum. ...
... Einstein extended Planck’s concept of quantization to electromagnetic waves. All electromagnetic radiation of frequency ƒ from any source can be considered a stream of quanta, now called photons. Each photon has an energy E and moves at the speed of light in a vacuum. ...
Physics 2018: Great Ideas in Science: The Physics Module Quantum
... In 1926 German physicist Erwin Schrödinger (1887–1961) published a paper on wave mechanics and what is now known as the Schrödinger equation. In this paper he gave a “derivation” of the wave equation for time independent systems, and showed that it gave the correct energy eigenvalues for the hydro ...
... In 1926 German physicist Erwin Schrödinger (1887–1961) published a paper on wave mechanics and what is now known as the Schrödinger equation. In this paper he gave a “derivation” of the wave equation for time independent systems, and showed that it gave the correct energy eigenvalues for the hydro ...
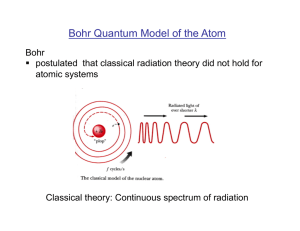
Bohr Quantum Model of the Atom
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
... § postulated that the electron orbital momentum is quantized Justification of Bohr’s postulates: comparison with experimental observations! ...
Let There Be Light
... A) Electromagnetic waves are longitudinal waves. B) Electromagnetic waves transfer energy through space. C) The existence of electromagnetic waves was predicted by Maxwell. D) Electromagnetic waves can propagate through a material substance. E) Electromagnetic waves do not require a physical medium ...
... A) Electromagnetic waves are longitudinal waves. B) Electromagnetic waves transfer energy through space. C) The existence of electromagnetic waves was predicted by Maxwell. D) Electromagnetic waves can propagate through a material substance. E) Electromagnetic waves do not require a physical medium ...
DUAL NATURE OF LIGHT WAVES A THEORETICAL PROOF
... different size and mass of particles. The corpuscle ...
... different size and mass of particles. The corpuscle ...
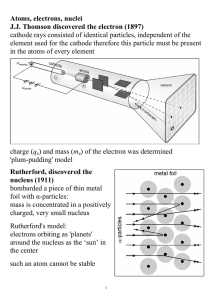
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
Lectures 7-9 - U of L Class Index
... It is inconsistent with experimental evidence (line spectra). The model implies that a hydrogen atom consists of an electron circling a proton. As such, the electron would be undergoing constant acceleration due to its constant change in direction. According to classical physics, acceleration of a c ...
... It is inconsistent with experimental evidence (line spectra). The model implies that a hydrogen atom consists of an electron circling a proton. As such, the electron would be undergoing constant acceleration due to its constant change in direction. According to classical physics, acceleration of a c ...
Lectures 7-9
... It is inconsistent with experimental evidence (line spectra). The model implies that a hydrogen atom consists of an electron circling a proton. As such, the electron would be undergoing constant acceleration due to its constant change in direction. According to classical physics, acceleration of a c ...
... It is inconsistent with experimental evidence (line spectra). The model implies that a hydrogen atom consists of an electron circling a proton. As such, the electron would be undergoing constant acceleration due to its constant change in direction. According to classical physics, acceleration of a c ...
Atomic Structure Review Part 1
... 5. Which subatomic particle was discovered by researchers working with a cathode ray tube? 6. What could Rutherford’s model not explain? ...
... 5. Which subatomic particle was discovered by researchers working with a cathode ray tube? 6. What could Rutherford’s model not explain? ...
Chapter 14 PowerPoint
... • This is a great equation for graphical analysis If given a table of f and Ek max or f and Vstop ...
... • This is a great equation for graphical analysis If given a table of f and Ek max or f and Vstop ...
Course summary for Unit 4 "Interactions of Light and
... Compare the momentum of photons and of particles of the same wavelength including calculations using , p = h/, Interpret electron diffraction patterns as evidence for the wave-like nature of matter expressed as the de Broglie wavelength = h/p; Momentum of A Photon In the Photon model, photons ha ...
... Compare the momentum of photons and of particles of the same wavelength including calculations using , p = h/, Interpret electron diffraction patterns as evidence for the wave-like nature of matter expressed as the de Broglie wavelength = h/p; Momentum of A Photon In the Photon model, photons ha ...
Quantum mechanics is the theory that we use to describe the
... In 1924, Louis De Broglie showed that matter itself had wavelike properties. He derived the equation, = h/p, that related a particle’s momentum with an associated wavelength. This equation tells us that all matter has wavelike properties, and must in some cases be thought of as existing as a wave, ...
... In 1924, Louis De Broglie showed that matter itself had wavelike properties. He derived the equation, = h/p, that related a particle’s momentum with an associated wavelength. This equation tells us that all matter has wavelike properties, and must in some cases be thought of as existing as a wave, ...
Chapter 2.2 and 7 Notes
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
QM_2_particles_ver2
... 1923: Classical mechanics “corresponds” to quantum system for BIG quantum numbers. ...
... 1923: Classical mechanics “corresponds” to quantum system for BIG quantum numbers. ...
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? E= nhc/λ; where E is the energy per minute and n is the number of photons per minute n= E λ/(hc); n =[ ...
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? E= nhc/λ; where E is the energy per minute and n is the number of photons per minute n= E λ/(hc); n =[ ...