Chapter 9 The Atom - Bakersfield College
... the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. The de Broglie waves of a moving object are in the ...
... the wave function (y). The square of the wave function (y2) is called the probability density. For a given object, the greater the probability density at a certain time and place, the greater the likelihood of finding the object there at that time. The de Broglie waves of a moving object are in the ...
Read more - Consumer Physics
... named after the physicist Max Planck who discovered it in 1900). To summarize the last two paragraphs- think about it in the following way: when a wave of light is propagating with wavelength , it is carried by many photons, each of them has exactly the same energy, ...
... named after the physicist Max Planck who discovered it in 1900). To summarize the last two paragraphs- think about it in the following way: when a wave of light is propagating with wavelength , it is carried by many photons, each of them has exactly the same energy, ...
Problem set 2 A - De Broglie wavelength B
... with q = k′ −k, k′ the wavevector scattered in a direction (θ, ϕ) and with k = k ′ because the scattering is elastic. When the energy of the collision is very small, k → 0 and therefore q → 0. The scattering amplitude f is then independent of the energy of the particle and isotropic. The scattering ...
... with q = k′ −k, k′ the wavevector scattered in a direction (θ, ϕ) and with k = k ′ because the scattering is elastic. When the energy of the collision is very small, k → 0 and therefore q → 0. The scattering amplitude f is then independent of the energy of the particle and isotropic. The scattering ...
Chapter 2 Particle properties of waves
... in separate quanta but was also carried by the waves in separate quanta. ...
... in separate quanta but was also carried by the waves in separate quanta. ...
Quantum and Nuclear Physics
... Schrödinger set out to develop an alternate formulation of quantum mechanics based on matter waves, à la de Broglie. At 36, he was somewhat older than his contemporaries but still succeeded in deriving the now famous 'Schrödinger Wave Equation.' The solution of the equation is known as a wave functi ...
... Schrödinger set out to develop an alternate formulation of quantum mechanics based on matter waves, à la de Broglie. At 36, he was somewhat older than his contemporaries but still succeeded in deriving the now famous 'Schrödinger Wave Equation.' The solution of the equation is known as a wave functi ...
Document
... Line Spectra and the Bohr Model Limitations of the Bohr Model • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can ha ...
... Line Spectra and the Bohr Model Limitations of the Bohr Model • Can only explain the line spectrum of hydrogen adequately. • Can only work for (at least) one electron atoms. • Cannot explain multi-lines with each color. • Electrons are not completely described as small particles. • Electrons can ha ...
Sample pages 1 PDF
... and Klaus von Klizing received the Nobel Prize in 1985 for his discovery of the quantum Hall effect. Standards for a quantum system of measurements based on quantum mechanical phenomena have been implemented in the past 25 years. Quantum phenomena are described in terms of concepts of quantum mechan ...
... and Klaus von Klizing received the Nobel Prize in 1985 for his discovery of the quantum Hall effect. Standards for a quantum system of measurements based on quantum mechanical phenomena have been implemented in the past 25 years. Quantum phenomena are described in terms of concepts of quantum mechan ...
What`s the big idea? - Perimeter Institute
... was spread out along its orbit, so instead of a rotating particle it was a rotating ring, then ...
... was spread out along its orbit, so instead of a rotating particle it was a rotating ring, then ...
Physics 228, Lecture 11 Monday, February 28, 2005 Bohr Model
... The same issue came up with spectial relativity, where we “threw out” the fundamental understanding of how coordinate systems were related, challenging Newtonian mechanics. But in that case it was pretty clear what was happening — the relativistic expressions, for example for momentum, reduced to th ...
... The same issue came up with spectial relativity, where we “threw out” the fundamental understanding of how coordinate systems were related, challenging Newtonian mechanics. But in that case it was pretty clear what was happening — the relativistic expressions, for example for momentum, reduced to th ...
Chapter 5
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the wave-particle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the o ...
... • DeBroglie, Einstein (and others) showed that electromagnetic radiation has properties of matter as well as waves. This is known as the wave-particle duality for light. • Wave-particle duality is perhaps one of the most confusing concepts in science, because it is so unlike anything we see in the o ...
Laws of Thermodynamics
... the ejected electrons was proportional to the frequency of the illuminating light. This showed that whatever was knocking the electrons out had an energy proportional to light frequency. The remarkable fact that the ejection energy was independent of the total energy of illumination showed that the ...
... the ejected electrons was proportional to the frequency of the illuminating light. This showed that whatever was knocking the electrons out had an energy proportional to light frequency. The remarkable fact that the ejection energy was independent of the total energy of illumination showed that the ...
Chemistry - Unit 6 What do you need to know?? This chapter is on
... Atoms are indestructible and unchangeable, so compounds, such as water and mercury calx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton’s theory implied that atoms bonded together, it would be more than 100 years befor ...
... Atoms are indestructible and unchangeable, so compounds, such as water and mercury calx, are formed when one atom chemically combines with other atoms. This was an extremely advanced concept for its time; while Dalton’s theory implied that atoms bonded together, it would be more than 100 years befor ...
kinetic energy of photoelectrons (eV)
... 1) Electrons are emitted from the metal only if the incident frequency is above a certain threshold frequency (fo). 2) Intensity (brightness of the light) had no effect on fo. No matter how bright the light, if it is below fo, no electrons are emitted. 3) Each metal has its own value of fo. ...
... 1) Electrons are emitted from the metal only if the incident frequency is above a certain threshold frequency (fo). 2) Intensity (brightness of the light) had no effect on fo. No matter how bright the light, if it is below fo, no electrons are emitted. 3) Each metal has its own value of fo. ...
The Nature of Electromagnetic Waves
... determined by the frequency. The speed of all electromagnetic waves through space is about 300,000 km/s. Answer the questions below based on the reading above, and on your knowledge of physics. 1. How is an electromagnetic wave different from a mechanical wave? ...
... determined by the frequency. The speed of all electromagnetic waves through space is about 300,000 km/s. Answer the questions below based on the reading above, and on your knowledge of physics. 1. How is an electromagnetic wave different from a mechanical wave? ...
Copenhagen Interpretation (of quantum physics)
... understanding of consciousness and the brain: “Current concepts of the mind-brain relationship involve a direct break with the long established materialist and behaviorist doctrine that has dominated neuroscience for many decades. Instead of renouncing or ignoring consciousness, the new interpretati ...
... understanding of consciousness and the brain: “Current concepts of the mind-brain relationship involve a direct break with the long established materialist and behaviorist doctrine that has dominated neuroscience for many decades. Instead of renouncing or ignoring consciousness, the new interpretati ...
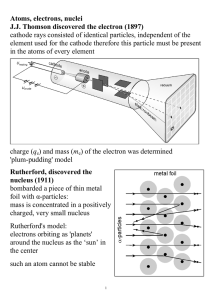
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... its momentum p = mv is given by the ‘shape’ of the function p = h/λ incorrect hypothesis for slower and faster ‘propagation’ of an electron in fact ψ(x,t) is a nonperiodic ...
... its momentum p = mv is given by the ‘shape’ of the function p = h/λ incorrect hypothesis for slower and faster ‘propagation’ of an electron in fact ψ(x,t) is a nonperiodic ...
Electromagnetic Waves - Galileo and Einstein
... few wavelengths) it has the familiar form shown above, the direction of propagation being directly away from the source. • For the wave shown above, generated by a vertical transmitting antenna, reception would be best with a vertical receiving antenna. The ...
... few wavelengths) it has the familiar form shown above, the direction of propagation being directly away from the source. • For the wave shown above, generated by a vertical transmitting antenna, reception would be best with a vertical receiving antenna. The ...