Slide 1
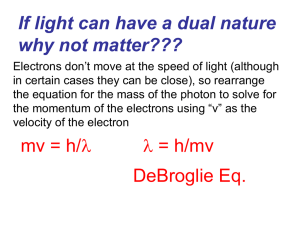
... Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
... Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
Who Invented the Copenhagen Interpretation? A Study in Mythology
... is independent of the means of observation. We are here faced with an epistemological problem quite new in natural philosophy, where all description of experience has so far been based on the assumption, already inherent in ordinary conventions of language, that it is possible to distinguish sharply ...
... is independent of the means of observation. We are here faced with an epistemological problem quite new in natural philosophy, where all description of experience has so far been based on the assumption, already inherent in ordinary conventions of language, that it is possible to distinguish sharply ...
The Transactional Interpretation
... ‘particle,’ such an electron, is? • The electron gets created in some state ‘Q’ • It could be in different positions a, b, c • Quantum theory just gives us probabilities for those positions: Prob(a|Q) or Prob(b|Q) or Prob(c|Q)….but no answer for why we only see 1 of them ...
... ‘particle,’ such an electron, is? • The electron gets created in some state ‘Q’ • It could be in different positions a, b, c • Quantum theory just gives us probabilities for those positions: Prob(a|Q) or Prob(b|Q) or Prob(c|Q)….but no answer for why we only see 1 of them ...
Louis de Broglie, the Father of Wave Mechanics
... of having particles reach a certain place through the two slits ...
... of having particles reach a certain place through the two slits ...
Quantum Mechanical Energy and You!
... position must be re-invented, as well as all secondary quantities. ...
... position must be re-invented, as well as all secondary quantities. ...
Anmeldeformular für Email
... to the classical phase space. The WF is a real valued function and in this respect compares with the classical probability density in phase space. Howev er, it is not alway s positive. Describing the dynamics of quantum mechanical transport proces s e s by coined or continuo us - time quantum walks ...
... to the classical phase space. The WF is a real valued function and in this respect compares with the classical probability density in phase space. Howev er, it is not alway s positive. Describing the dynamics of quantum mechanical transport proces s e s by coined or continuo us - time quantum walks ...
Quantum Physics - Particle Physics and Particle Astrophysics
... • Impossible, even in principle, to know position and momentum of particle exactly and simultaneously ...
... • Impossible, even in principle, to know position and momentum of particle exactly and simultaneously ...
Lecture notes, part 6
... Since most spectroscopy techniques operate on a sample which is a sizeable fraction of Avogadro’s number, we can invoke some statistical methods to understand and predict the peak intensities. Fundamental Postulate of Statistical Mechanics: Given an isolated system at equilibrium, it is found with e ...
... Since most spectroscopy techniques operate on a sample which is a sizeable fraction of Avogadro’s number, we can invoke some statistical methods to understand and predict the peak intensities. Fundamental Postulate of Statistical Mechanics: Given an isolated system at equilibrium, it is found with e ...
CHEMISTRY CHAPTER 4 – QUANTUM MECHANICS
... 3. Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. 4. Describe the Bohr model of the hydrogen atom. 5. Discuss Louis de Broglie’s role in the development of the quantum model of the atom. 6. Compare and contrast ...
... 3. Discuss the significance of the photoelectric effect and the line-emission spectrum of hydrogen to the development of the atomic model. 4. Describe the Bohr model of the hydrogen atom. 5. Discuss Louis de Broglie’s role in the development of the quantum model of the atom. 6. Compare and contrast ...
Document
... Which of the following phenomena could be explained by classical physics and did not require a quantum hypothesis in order to make theory agree with experiment? ...
... Which of the following phenomena could be explained by classical physics and did not require a quantum hypothesis in order to make theory agree with experiment? ...
SuperString Theory
... The only theory which can change the universe. Is Einstein wrong? The smallest, but the biggest. ...
... The only theory which can change the universe. Is Einstein wrong? The smallest, but the biggest. ...
Lectuer 15
... - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – fold degeneracy in the absence of a magnetic field is spli ...
... - The z component of the angular momentum is determined completely by m through L z = m ħ. - The quantum number m is called the magnetic quantum number because the energy of a hydrogen atom in a magnetic field depends on m. - The (2 Ɩ + 1) – fold degeneracy in the absence of a magnetic field is spli ...
Postulate 1
... mechanics. Some of this material may be familiar from mathematics courses. The “eigenfunctions” that are useful in describing particles with wave properties are of familiar form (and, in part, predictable?). ...
... mechanics. Some of this material may be familiar from mathematics courses. The “eigenfunctions” that are useful in describing particles with wave properties are of familiar form (and, in part, predictable?). ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... Every microscope has the limit (the so-called diffraction limit) of observing a point like particle with a width of ∆x = λ / sinθ . This is then the accuracy With which we know the particles position ...
... Every microscope has the limit (the so-called diffraction limit) of observing a point like particle with a width of ∆x = λ / sinθ . This is then the accuracy With which we know the particles position ...
Quantum Information (QI) - BYU Physics and Astronomy
... Sagawa and Yoshida: Fundamentals of QI Valerio Scarani: Six Quantum Pieces Vlatko Vedral: Introduction to QI Gennnaro Auletta: Foundation and Interpretation of Quantum Mechanics Benenti, Casati, and Strini: Principles of Q Computation and Information Eugen Merzbacher: Quantum Mechanics Nielsen and C ...
... Sagawa and Yoshida: Fundamentals of QI Valerio Scarani: Six Quantum Pieces Vlatko Vedral: Introduction to QI Gennnaro Auletta: Foundation and Interpretation of Quantum Mechanics Benenti, Casati, and Strini: Principles of Q Computation and Information Eugen Merzbacher: Quantum Mechanics Nielsen and C ...
Quantum Mechanics
... Nothing is more important about the quantum principle that this, that it destroys the concept of the world as sitting ‘out there.’ … the measurement changes the state of the electron. The universe will never afterwards be the same. To describe what has happened, one has to cross out that old word ‘o ...
... Nothing is more important about the quantum principle that this, that it destroys the concept of the world as sitting ‘out there.’ … the measurement changes the state of the electron. The universe will never afterwards be the same. To describe what has happened, one has to cross out that old word ‘o ...
Heisenberg uncertainty principle
... poison is then subject to the probabilistic decay of a radioactive isotope. If the isotope decays, the poison is released. If no decay occurs, the poison is not released. The result is that the cat is in a superposition of states between being dead, and being alive. This is very unintuitive. ...
... poison is then subject to the probabilistic decay of a radioactive isotope. If the isotope decays, the poison is released. If no decay occurs, the poison is not released. The result is that the cat is in a superposition of states between being dead, and being alive. This is very unintuitive. ...
Problem set 6
... of each Fourier component of a matter wave ψ(x, t) was given by ei(kx−ω(k)t) corresponding to a right moving wave if k, ω(k) were of the same sign. We could equally well have considered the time evolution ei(kx+ω(k)t) . We do this here. Write down an expression for φ(x, t) for this ‘left moving’ wav ...
... of each Fourier component of a matter wave ψ(x, t) was given by ei(kx−ω(k)t) corresponding to a right moving wave if k, ω(k) were of the same sign. We could equally well have considered the time evolution ei(kx+ω(k)t) . We do this here. Write down an expression for φ(x, t) for this ‘left moving’ wav ...