CHM 441: QUANTUM CHEMISTRY
... which marks the beginning of quantum mechanics applied to atoms, but was unable to describe atoms with more than one electron. ...
... which marks the beginning of quantum mechanics applied to atoms, but was unable to describe atoms with more than one electron. ...
Physics 7910: HW # 03.
... Find the ordering momentum and the energy of the ground state configuration as a function of the dimensionless ratio w = −J2 /J1 in the full possible range 0 ≤ w ≤ ∞. [The problem is motivated by recently discovered frustrated ferromagnets LiCuVO4 and LiCu2 O2 .] ~ and the ground state energy E0 of ...
... Find the ordering momentum and the energy of the ground state configuration as a function of the dimensionless ratio w = −J2 /J1 in the full possible range 0 ≤ w ≤ ∞. [The problem is motivated by recently discovered frustrated ferromagnets LiCuVO4 and LiCu2 O2 .] ~ and the ground state energy E0 of ...
Operators and meaning of wave function
... indicated at least nine different interpretations used in quantum theory. However, we must say that different interpretations can not be distinguished by purely scientific methods. When the different interpretations give the same experimental results, we have not a different theory. In this paper we ...
... indicated at least nine different interpretations used in quantum theory. However, we must say that different interpretations can not be distinguished by purely scientific methods. When the different interpretations give the same experimental results, we have not a different theory. In this paper we ...
SAND Quantum Theory of What
... Awareness is essential to the arising of the mind. 4. Quantum theory would describe the arising of subjective mind states (not brain states) in Awareness, plus the subjective process of decision making. 5. While a big step forward, the interpretation of Christopher Fuchs is a theory of subjective mi ...
... Awareness is essential to the arising of the mind. 4. Quantum theory would describe the arising of subjective mind states (not brain states) in Awareness, plus the subjective process of decision making. 5. While a big step forward, the interpretation of Christopher Fuchs is a theory of subjective mi ...
Brief introduction to quantum mechanics
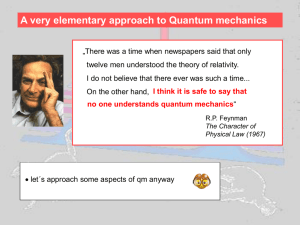
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
... A very elementary approach to Quantum mechanics „There was a time when newspapers said that only twelve men understood the theory of relativity. I do not believe that there ever was such a time... On the other hand, I think it is safe to say that no one understands quantum mechanics“ R.P. Feynman Th ...
Physics 411: Introduction to Quantum Mechanics
... Homework assignments will be handed out once per week and must be turned it one week later, same day. We typically have ten homework assignments per semester. Homework is graded on a scale from 1 (= poor) to 3 (very good or excellent). Midterms will be split into a take home problem and a test in cl ...
... Homework assignments will be handed out once per week and must be turned it one week later, same day. We typically have ten homework assignments per semester. Homework is graded on a scale from 1 (= poor) to 3 (very good or excellent). Midterms will be split into a take home problem and a test in cl ...
The Parable of the Three Umpires
... ability to capture or “enframe” in language. An electron, for example, can exhibit both “wave” or “particle” behaviour depending on how we interact with it. ...
... ability to capture or “enframe” in language. An electron, for example, can exhibit both “wave” or “particle” behaviour depending on how we interact with it. ...
Quantum Model Worksheet
... 5. Complete the following table to indicate the total number of orbitals in each energy level (n). In the remaining columns, specify how many of those orbitals are s, p, d, and f. Level n ...
... 5. Complete the following table to indicate the total number of orbitals in each energy level (n). In the remaining columns, specify how many of those orbitals are s, p, d, and f. Level n ...
Quantum Model Worksheet
... 5. Complete the following table to indicate the total number of orbitals in each energy level (n). In the remaining columns, specify how many of those orbitals are s, p, d, and f. Level n ...
... 5. Complete the following table to indicate the total number of orbitals in each energy level (n). In the remaining columns, specify how many of those orbitals are s, p, d, and f. Level n ...
The Blind Men and the Quantum
... (Though all of them were blind), That each by observation, Might satisfy his mind. . The First approached the Elephant, And happening to fall, Against his broad and sturdy side, At once began to bawl: “God bless me! but the Elephant, Is very like a wall!” The Second, feeling of the tusk, Cried, “Ho! ...
... (Though all of them were blind), That each by observation, Might satisfy his mind. . The First approached the Elephant, And happening to fall, Against his broad and sturdy side, At once began to bawl: “God bless me! but the Elephant, Is very like a wall!” The Second, feeling of the tusk, Cried, “Ho! ...
Objective of the course Aim of the course is to introduce the basic
... Aim of the course is to introduce the basic notions of non-relativistic quantum mechanics and its interpretation. At the end of the course the students should: 1) have understood the definition of physical state and the superposition principle in quantum mechanics, the definition of physical observa ...
... Aim of the course is to introduce the basic notions of non-relativistic quantum mechanics and its interpretation. At the end of the course the students should: 1) have understood the definition of physical state and the superposition principle in quantum mechanics, the definition of physical observa ...
Chemistry 871/671/495, Structure and Bonding
... mechanical laws, which are quite different from classical mechanics that dictate our macroscopic world. To understand the structure of molecules and their reactivity, one has no choice but to rely on quantum mechanics. In this course, we will introduce quantum mechanical principles and their applica ...
... mechanical laws, which are quite different from classical mechanics that dictate our macroscopic world. To understand the structure of molecules and their reactivity, one has no choice but to rely on quantum mechanics. In this course, we will introduce quantum mechanical principles and their applica ...
p 2 ! πλ=
... This makes it clear that in quantum mechanics probability statements are often obtained, whereas in classical mechanics the location of a particle can be determined exactly. The Schrodinger Equation Solution to the calculation and interpretation of Ψ provided by Schrodinger in 1925: Motion of electr ...
... This makes it clear that in quantum mechanics probability statements are often obtained, whereas in classical mechanics the location of a particle can be determined exactly. The Schrodinger Equation Solution to the calculation and interpretation of Ψ provided by Schrodinger in 1925: Motion of electr ...
슬라이드 1
... small flask of hydrocyanic acid. If one has left this entire system to itself for an hour, one would say that the cat still lives if meanwhile no atom has decayed. The psi-function of the entire system would express this by having in it the living and dead cat (pardon the expression) mixed or smeare ...
... small flask of hydrocyanic acid. If one has left this entire system to itself for an hour, one would say that the cat still lives if meanwhile no atom has decayed. The psi-function of the entire system would express this by having in it the living and dead cat (pardon the expression) mixed or smeare ...
Document
... Schrödinger Equation Since the solution to the Schrödinger equation is supposed to represent a single particle, the total probability of finding that particle anywhere in ...
... Schrödinger Equation Since the solution to the Schrödinger equation is supposed to represent a single particle, the total probability of finding that particle anywhere in ...
Quantum mechanics in electronics
... which occupies the value 0, 1 or both simultaneously • Concepts used : entanglement , superposition ...
... which occupies the value 0, 1 or both simultaneously • Concepts used : entanglement , superposition ...
REVIEW OF WAVE MECHANICS
... measurement, after the measurement it has been “reduced” or “collapsed” to one eigenfunction (assuming that we have performed a perfect ‘noise-free’ experiment and found a definite value for the measured quantity). ...
... measurement, after the measurement it has been “reduced” or “collapsed” to one eigenfunction (assuming that we have performed a perfect ‘noise-free’ experiment and found a definite value for the measured quantity). ...
Kepler`s elliptic orbits in wave mechanics, and problems with the de
... Santa Cruz www.physics.ucsc\~michael ...
... Santa Cruz www.physics.ucsc\~michael ...