PH5015 - Applications of Quantum Physics
... Signatures of BEC and Fermi gases. Matter wave interference. Wave-particle duality studies. Charged ion trapping. Studies of laser cooled ions in traps. Quantum jumps. Atom lasers. The second half of the course explores the statistics of light: coherence. First and second order correlation functions ...
... Signatures of BEC and Fermi gases. Matter wave interference. Wave-particle duality studies. Charged ion trapping. Studies of laser cooled ions in traps. Quantum jumps. Atom lasers. The second half of the course explores the statistics of light: coherence. First and second order correlation functions ...
Instructor: Dr. Ju Xin
... References: “Modern Physics for Scientists and Engineers” By Stephen T. Thornton, Andrew F. Rex - Thomson, Brooks/Cole (2006) - Hardback - 672 pages - ISBN 0534417817 Coverage: The first 10 chapters form the core contents of modern and atomic physics. We will selectively cover most of these chapters ...
... References: “Modern Physics for Scientists and Engineers” By Stephen T. Thornton, Andrew F. Rex - Thomson, Brooks/Cole (2006) - Hardback - 672 pages - ISBN 0534417817 Coverage: The first 10 chapters form the core contents of modern and atomic physics. We will selectively cover most of these chapters ...
Chapter 10 Lattice Heat Capacity - Physics | Oregon State University
... What is universally observed is C ∼ T 3 . Einstein was aware that a single oscillator frequency model was bound to be inadequate and he did try to improve upon it, without success. His primary objective, however, was to apply quantum theory and show that it explained several poorly understood phenom ...
... What is universally observed is C ∼ T 3 . Einstein was aware that a single oscillator frequency model was bound to be inadequate and he did try to improve upon it, without success. His primary objective, however, was to apply quantum theory and show that it explained several poorly understood phenom ...
Quantum emergence and role of the zero-point field
... When the particles have a common res. frequency, ...
... When the particles have a common res. frequency, ...
Document
... 1) CLASSICAL WAVE THEORY We have seen that electromagnetic energy (such as light) behaves as a continuous wave - It can be reflected, refracted and diffracted. More importantly, it can produce interference (which is the test for wave motion). ...
... 1) CLASSICAL WAVE THEORY We have seen that electromagnetic energy (such as light) behaves as a continuous wave - It can be reflected, refracted and diffracted. More importantly, it can produce interference (which is the test for wave motion). ...
Irradiance of Electromagnetic Radiation
... 1) CLASSICAL WAVE THEORY We have seen that electromagnetic energy (such as light) behaves as a continuous wave - It can be reflected, refracted and diffracted. More importantly, it can produce interference (which is the test for wave motion). ...
... 1) CLASSICAL WAVE THEORY We have seen that electromagnetic energy (such as light) behaves as a continuous wave - It can be reflected, refracted and diffracted. More importantly, it can produce interference (which is the test for wave motion). ...
Who Invented the Copenhagen Interpretation? A Study in Mythology
... observation. This assumption is not only fully justified by all everyday experience but even constitutes the whole basis of classical physics. . . . As soon as we are dealing, however, with phenomena like individual atomic processes which, due to their very nature, are essentially determined by the ...
... observation. This assumption is not only fully justified by all everyday experience but even constitutes the whole basis of classical physics. . . . As soon as we are dealing, however, with phenomena like individual atomic processes which, due to their very nature, are essentially determined by the ...
Reverse Engineer Relativity, Quantum Mechanics and the Standard
... is now long overdue, but this time around, it is some of Einstein's own foundational assumptions that have to be discarded. Physics was not invented (or discovered) in one go, but was built, one assumption on top of another. Seven of what I consider the most basic concepts (apart from questions in c ...
... is now long overdue, but this time around, it is some of Einstein's own foundational assumptions that have to be discarded. Physics was not invented (or discovered) in one go, but was built, one assumption on top of another. Seven of what I consider the most basic concepts (apart from questions in c ...
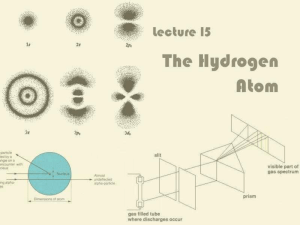
Chapter 7. The Quantum-Mechanical Model of the Atom 100
... Know and understand how atomic spectroscopy defines the energy levels of electrons in the hydrogen atom. Calculate the energies and wavelengths of emitted and absorbed photons for hydrogen. ...
... Know and understand how atomic spectroscopy defines the energy levels of electrons in the hydrogen atom. Calculate the energies and wavelengths of emitted and absorbed photons for hydrogen. ...
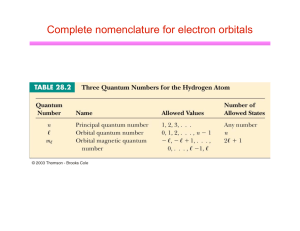
Complete nomenclature for electron orbitals
... Stimulated emission l Suppose an atom is in an excited state E2 and a photon with energy hf=E2-E1 is incident on the atom l Incoming photon of this energy increases the probability of the atom returning to the ground state emitting a photon of the same energy hf as the incident photon and in phase ...
... Stimulated emission l Suppose an atom is in an excited state E2 and a photon with energy hf=E2-E1 is incident on the atom l Incoming photon of this energy increases the probability of the atom returning to the ground state emitting a photon of the same energy hf as the incident photon and in phase ...
Learning station IV: Wave Particle Duality
... In the 19th century it became clear that light was a wave phenomenon: a wave of electric and magnetic fields. But it soon turned out that this did not exact ly revealed the true nature of light. Let us therefore look back to the double slit experiment for light: We know that light that passes two sl ...
... In the 19th century it became clear that light was a wave phenomenon: a wave of electric and magnetic fields. But it soon turned out that this did not exact ly revealed the true nature of light. Let us therefore look back to the double slit experiment for light: We know that light that passes two sl ...
Quantum Mechanics
... - When the experiment is repeated with large number of particles the diffraction pattern is observed. ...
... - When the experiment is repeated with large number of particles the diffraction pattern is observed. ...
Undergraduate Laboratories Using Correlated Photons: Experiments on the Fundamentals of Quantum Physics
... photon may randomly pick any one of the two paths in going through the interferometer. However, quantum mechanics says that the photon takes both paths. One may ask, “How do we know the difference?” Quantum mechanics explains that if the paths are indistinguishable, then a measurement of the photon ...
... photon may randomly pick any one of the two paths in going through the interferometer. However, quantum mechanics says that the photon takes both paths. One may ask, “How do we know the difference?” Quantum mechanics explains that if the paths are indistinguishable, then a measurement of the photon ...
Does Quantum Mechanics Make Sense?
... Photoelectric effect: photons as particles Need to know about the nature of the probability amplitude waves, how they combine, and what happens when you make a measurement. ...
... Photoelectric effect: photons as particles Need to know about the nature of the probability amplitude waves, how they combine, and what happens when you make a measurement. ...
No Slide Title
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.