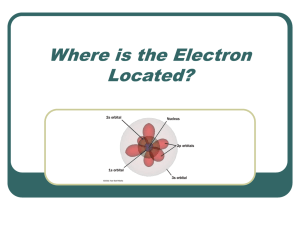
Properties of wave functions (Text 5.1)
... Classical mechanics turns out to be just an approximate version of quantum mechanics. The certainties of classical mechanics are illusory, and their apparent agreement with experiment occurs because ordinary objects consist of so many individual atoms that departures from average behavior are unnoti ...
... Classical mechanics turns out to be just an approximate version of quantum mechanics. The certainties of classical mechanics are illusory, and their apparent agreement with experiment occurs because ordinary objects consist of so many individual atoms that departures from average behavior are unnoti ...
Section 1 Notes
... observing how often the water level rises and falls at a given point (the post). Frequency is defined as the number of waves that pass a given point in a specific time, usually one second: f = waves / time. (waves/second). One wave/second is called a hertz (Hz). 1 wave / s = 1 hertz (Hz) Frequency a ...
... observing how often the water level rises and falls at a given point (the post). Frequency is defined as the number of waves that pass a given point in a specific time, usually one second: f = waves / time. (waves/second). One wave/second is called a hertz (Hz). 1 wave / s = 1 hertz (Hz) Frequency a ...
CHAPTER 11: Through the Looking Glass
... A troubling inconsistency had escaped the attention of most classical physicists: physics described Nature as “schizophrenic.” Newtonian mechanics dealt with particles. Maxwellian electromagnetics dealt with waves. But particles and waves are mutually exclusive. Whereas particles are localized in s ...
... A troubling inconsistency had escaped the attention of most classical physicists: physics described Nature as “schizophrenic.” Newtonian mechanics dealt with particles. Maxwellian electromagnetics dealt with waves. But particles and waves are mutually exclusive. Whereas particles are localized in s ...
Part One: Light Waves, Photons, and Bohr Theory A. The Wave
... Rydberg found an equation that reproduces the λ of the all lines in Hydrogen emission spectrum, including ultraviolet and infrared: ...
... Rydberg found an equation that reproduces the λ of the all lines in Hydrogen emission spectrum, including ultraviolet and infrared: ...
Quantum Memories at Room-Temperature Supervisors: Dr Dylan
... For the Master’s project, we are proposing an investigation into a new noise-suppression technique in our lambda Raman quantum memory. This will be demonstration of a new protocol: a quantum Zeno noise suppression technique to kill a noise-process prohibits quantum operation, a process known as four ...
... For the Master’s project, we are proposing an investigation into a new noise-suppression technique in our lambda Raman quantum memory. This will be demonstration of a new protocol: a quantum Zeno noise suppression technique to kill a noise-process prohibits quantum operation, a process known as four ...
Introduction to Quantum Physics
... In the latter part of the 19th century experiments show that light incident on certain metallic surfaces caused electrons to be emitted from the surfaces. This phenomenon is known as photoelectric effect and the emitted electrons are called photoelectrons. In the below figure when light strikes the ...
... In the latter part of the 19th century experiments show that light incident on certain metallic surfaces caused electrons to be emitted from the surfaces. This phenomenon is known as photoelectric effect and the emitted electrons are called photoelectrons. In the below figure when light strikes the ...
L 35 Modern Physics [1] Modern Physics
... and the Bohr Atom • Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a ...
... and the Bohr Atom • Niels Bohr, a Danish physicist, used the quantum concept to explain the nature of the atom. • Recall that the orbiting electrons, according to classical ideas, should very quickly radiate away all of its energy • If this were so, then we would observe that atoms emit light over a ...
L34 - University of Iowa Physics
... Newton’s laws also fail at high velocities Electron Kinetic Energy ...
... Newton’s laws also fail at high velocities Electron Kinetic Energy ...
Atomic and Molecular Physics for Physicists Ben-Gurion University of the Negev
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
... angular momentum J, and as JZ=LZ+SZ, and as, when calculating the distances and therefore the forces one has to take into account that g for the orbital motion is gL=1 while for the spin is gS=2, we will have the following forces acting on the atoms: F(LZ=+1, SZ=+1/2), F(LZ=+0, SZ=+1/2), F(LZ=-1, SZ ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.














![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/000679677_1-b925cf8c8f031b0f2b0c09a806312d20-300x300.png)
![L 35 Modern Physics [1] Modern Physics](http://s1.studyres.com/store/data/001558975_1-84d6e03bc786b63795533f59711ce2f4-300x300.png)