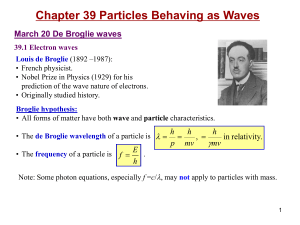
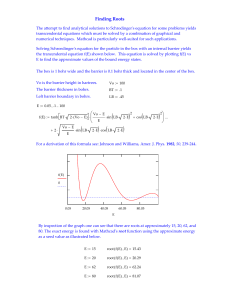
energy levels
... • An absorption spectrum is obtained by passing a white light from a continuous source ...
... • An absorption spectrum is obtained by passing a white light from a continuous source ...
Problem set #1 - U.C.C. Physics Department
... 4) Let us assume that a light bulb emits a monochromatic yellow light, at wavelength λ = 600 nm. You are standing 3 meters away from a 60 W light bulb, and looks at it. Calculate the number of photons that enter your eyes each second. You can assume that the light bulb’s emission is spherical, and t ...
... 4) Let us assume that a light bulb emits a monochromatic yellow light, at wavelength λ = 600 nm. You are standing 3 meters away from a 60 W light bulb, and looks at it. Calculate the number of photons that enter your eyes each second. You can assume that the light bulb’s emission is spherical, and t ...
There are 4 quantum numbers. - 12S7F-note
... The principle quantum number [n] refers to the electron shell that the electron exists in. The angular momentum number [l] is the orbital of the electron i.e. the s-orbital is represented by 0, the p-orbital by 1, the d-orbital by 2 and so on. The magnetic quantum number [ml] is the sub-orbital or c ...
... The principle quantum number [n] refers to the electron shell that the electron exists in. The angular momentum number [l] is the orbital of the electron i.e. the s-orbital is represented by 0, the p-orbital by 1, the d-orbital by 2 and so on. The magnetic quantum number [ml] is the sub-orbital or c ...
Molecular or Stringy Photon, One or Few
... indicates this important fact that our current traditional divisions such as second , minutes, hours and ... aren't deducted by human beings; instead they are related to high-intelligent creatures which they are using from an advanced geometry and mathematics and they have transferred knowledge of ...
... indicates this important fact that our current traditional divisions such as second , minutes, hours and ... aren't deducted by human beings; instead they are related to high-intelligent creatures which they are using from an advanced geometry and mathematics and they have transferred knowledge of ...
Class 23_270_11
... or slit#2 must be wrong. Electron must be going through both slits at the same time !!! to exhibit interference ...
... or slit#2 must be wrong. Electron must be going through both slits at the same time !!! to exhibit interference ...
class slides for Chapter 38
... wave that fills up the space between source and screen and then vanishes in a photon absorption at some point on the screen, with a transfer of energy and momentum to the screen at that point. We cannot predict where this transfer will occur (where a photon will be detected) for any given photon ori ...
... wave that fills up the space between source and screen and then vanishes in a photon absorption at some point on the screen, with a transfer of energy and momentum to the screen at that point. We cannot predict where this transfer will occur (where a photon will be detected) for any given photon ori ...
From E = mc2 to E = mc2/22—A Short Account
... discussion between Einstein and himself in which Heisenberg thought to include only directly observable quantities in a theory and thought that this is what guided Einstein to his relativity but Einstein vehemently disagreed [3]. Clearly this is again Einstein’s principle stance that it is the theor ...
... discussion between Einstein and himself in which Heisenberg thought to include only directly observable quantities in a theory and thought that this is what guided Einstein to his relativity but Einstein vehemently disagreed [3]. Clearly this is again Einstein’s principle stance that it is the theor ...
The quantum mechanics of photon addition and subtraction
... composed of a small number of photons, the effect is dramatic and may even completely change the characteristics of the field. For example, let us take an initial single-mode squeezed state generated by ‘degenerate’ parametric down-conversion, which is an optical nonlinear process that converts a pu ...
... composed of a small number of photons, the effect is dramatic and may even completely change the characteristics of the field. For example, let us take an initial single-mode squeezed state generated by ‘degenerate’ parametric down-conversion, which is an optical nonlinear process that converts a pu ...
January 1999
... J99M.2—Particle Rolling on a Hyperboloid Problem A particle of mass m moves on the surface x2 + y 2 − z 2 = R2 , subject to a uniform force due to gravity g directed along the negative z axis. Deduce the frequency of small oscillations about orbits that lie in a plane normal to the z axis. For what ...
... J99M.2—Particle Rolling on a Hyperboloid Problem A particle of mass m moves on the surface x2 + y 2 − z 2 = R2 , subject to a uniform force due to gravity g directed along the negative z axis. Deduce the frequency of small oscillations about orbits that lie in a plane normal to the z axis. For what ...
Environmental Sensors Photosynthetic Photon Flux Sensor
... quantify potential for plant photosynthesis by measuring active radiation in the wavelength ranges strongly correlated with plant growth. The sensor is calibrated for use in sunlight, and an innovative blue lens improves the accuracy of measurements. The pigments in the lens filter the incoming ligh ...
... quantify potential for plant photosynthesis by measuring active radiation in the wavelength ranges strongly correlated with plant growth. The sensor is calibrated for use in sunlight, and an innovative blue lens improves the accuracy of measurements. The pigments in the lens filter the incoming ligh ...
Chapter 7 Worksheet November 1
... C. The light waves bend around an object D. The light waves disperse into their component colors 8. What might the problem be if our retina could detect low frequency electromagnetic radiation? ...
... C. The light waves bend around an object D. The light waves disperse into their component colors 8. What might the problem be if our retina could detect low frequency electromagnetic radiation? ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.