L35 - University of Iowa Physics
... = 350 nm (nanometers) have compared to a photon of wavelength = 700 nm? • Solution: The shorter wavelength photon has the higher frequency. The 350 nm photon has twice the frequency as the 700 nm photon. Therefore, the 350 nm photon has twice the energy as the 700 nm photon. ...
... = 350 nm (nanometers) have compared to a photon of wavelength = 700 nm? • Solution: The shorter wavelength photon has the higher frequency. The 350 nm photon has twice the frequency as the 700 nm photon. Therefore, the 350 nm photon has twice the energy as the 700 nm photon. ...
You are going to read the chapter at home.
... Completeness: We can expand the Nparticle wave function as a product of single-particle wave functions __ ...
... Completeness: We can expand the Nparticle wave function as a product of single-particle wave functions __ ...
Chapter 7: ELECTRONS IN ATOMS AND
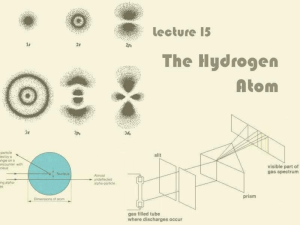
... • Although it successfully described the line spectrum of hydrogen and other one-electron systems, it failed to accurately describe the spectra of multi-electron atoms. • The Bohr model was soon scrapped in favor of the Quantum Mechanical model, although the vocabulary of the Bohr model persists. • ...
... • Although it successfully described the line spectrum of hydrogen and other one-electron systems, it failed to accurately describe the spectra of multi-electron atoms. • The Bohr model was soon scrapped in favor of the Quantum Mechanical model, although the vocabulary of the Bohr model persists. • ...
Jan. 23, 2006
... state, and that the energies of those photons are equal to Planck's constant times their frequency (E = hν), then we have ...
... state, and that the energies of those photons are equal to Planck's constant times their frequency (E = hν), then we have ...
energy
... element is the set of frequencies of the electromagnetic waves emitted by atoms of the element. ...
... element is the set of frequencies of the electromagnetic waves emitted by atoms of the element. ...
Otto Stern and the discovery of space quantization
... in one way or another since on leaving the source they were arranged quite statistically. There was no way by which those in the negative direction could gain or lose energy. In fact, the whole thing ...
... in one way or another since on leaving the source they were arranged quite statistically. There was no way by which those in the negative direction could gain or lose energy. In fact, the whole thing ...
Old Physics GRE Problems Based on content from Chapter 2 of your
... E. It cannot be determined unless a gauge transformation is also specified. 4. If a newly discovered particle X moves with a speed equal to the speed of light in a vacuum, then which of the following must be true? A. The rest mass of X is zero. B. The spin of X equals the spin of a photon. C. The ch ...
... E. It cannot be determined unless a gauge transformation is also specified. 4. If a newly discovered particle X moves with a speed equal to the speed of light in a vacuum, then which of the following must be true? A. The rest mass of X is zero. B. The spin of X equals the spin of a photon. C. The ch ...
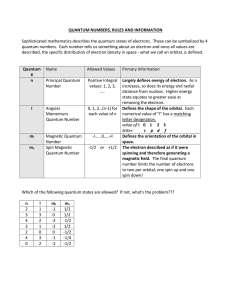
What is the quantum state?
... • Non-orthogonal quantum states cannot reliably be distinguished – just like probability distributions. • Quantum states are exponential in the number of systems – just ...
... • Non-orthogonal quantum states cannot reliably be distinguished – just like probability distributions. • Quantum states are exponential in the number of systems – just ...
homework 2, due October 3rd
... Consider a particle described at some particular instant of time by the wave function ψ(x) = Ae−ax . 1. Determine A so ψ is normalized. 2. Compute hxi, hx2 i and σx2 = h(x − hxi)2 i. 3. Compute hpi, hp2 i and σp2 = h(p − hpi)2 i. 4. Show that by changing a one can make either σx2 or σp2 small, but n ...
... Consider a particle described at some particular instant of time by the wave function ψ(x) = Ae−ax . 1. Determine A so ψ is normalized. 2. Compute hxi, hx2 i and σx2 = h(x − hxi)2 i. 3. Compute hpi, hp2 i and σp2 = h(p − hpi)2 i. 4. Show that by changing a one can make either σx2 or σp2 small, but n ...
Document
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
... • Quantum physics explains the energy levels of atoms with enormous accuracy. This is possible, since these levels have long lifetime (uncertainty relation for E, t). • Radiation from atoms and molecules enables the most accurate time and length measurements: Atomic clocks • Quantum physics explai ...
Relation and quantum gravity in the light of Simondon and
... relation totale et relation pure.»1 (Bachelard 1929) In a most unique and creative way, extending Bachelard’s analyses, Gilbert Simondon (1924-1989) has developed an original philosophy of individuation where he states (as early as 1964) the necessity to give an ontological value to the concept of r ...
... relation totale et relation pure.»1 (Bachelard 1929) In a most unique and creative way, extending Bachelard’s analyses, Gilbert Simondon (1924-1989) has developed an original philosophy of individuation where he states (as early as 1964) the necessity to give an ontological value to the concept of r ...
Slides from lecture 4.
... Unlike sound waves or water waves, matter waves are not composed of some material substance. Matter waves are simply measures of probability. So, in principle one cannot be certain what any given particle will do exactly; only betting odds can be given. ...
... Unlike sound waves or water waves, matter waves are not composed of some material substance. Matter waves are simply measures of probability. So, in principle one cannot be certain what any given particle will do exactly; only betting odds can be given. ...
Bohr–Einstein debates

The Bohr–Einstein debates were a series of public disputes about quantum mechanics between Albert Einstein and Niels Bohr. Their debates are remembered because of their importance to the philosophy of science. An account of the debates was written by Bohr in an article titled ""Discussions with Einsteinon Epistemological Problems in Atomic Physics"". Despite their differences of opinion regarding quantum mechanics, Bohr and Einstein had a mutual admiration that was to last the rest of their lives.The debates represent one of the highest points of scientific research in the first half of the twentieth century because it called attention to an element of quantum theory, quantum non-locality, which is absolutely central to our modern understanding of the physical world. The consensus view of professional physicists has been that Bohr proved victorious, and definitively established the fundamental probabilistic character of quantum measurement.




![L 35 Modern Physics [1] - University of Iowa Physics](http://s1.studyres.com/store/data/001147028_1-f00aa7577568b42bc32948cbade9023a-300x300.png)

















