* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 13: Heisenberg and Uncertainty
X-ray photoelectron spectroscopy wikipedia , lookup
Interpretations of quantum mechanics wikipedia , lookup
Symmetry in quantum mechanics wikipedia , lookup
Hidden variable theory wikipedia , lookup
Quantum state wikipedia , lookup
Renormalization group wikipedia , lookup
Werner Heisenberg wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Coherent states wikipedia , lookup
Elementary particle wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Renormalization wikipedia , lookup
Atomic orbital wikipedia , lookup
Copenhagen interpretation wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
EPR paradox wikipedia , lookup
Hydrogen atom wikipedia , lookup
Atomic theory wikipedia , lookup
Particle in a box wikipedia , lookup
Double-slit experiment wikipedia , lookup
Matter wave wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
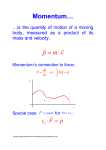
Lecture 13: Heisenberg and Uncertainty Determinism of Classical Mechanics Suppose the positions and speeds of all particles in the universe are measured to sufficient accuracy at a particular instant in time It is possible to predict the motions of every particle at any time in the future (or in the past for that matter) “An intelligent being knowing, at a given instant of time, all forces acting in nature, as well as the momentary positions of all things of which the universe consists, would be able to comprehend the motions of the largest bodies of the world and those of the smallest atoms in one single formula, provided it were sufficiently powerful to subject all the data to analysis; to it, nothing would be uncertain, both future and past would be present before its eyes.” Pierre Simon Laplace Role of an Observer The observer is objective and passive Physical events happen independently of whether there is an observer or not This is known as objective reality Double-Slit Experiment: cannot predict where electron would land Double-Slit Experiment: act of observation affects behaviour of electron Role of an Observer in Quantum Mechanics The observer is not objective and passive The act of observation changes the physical system irrevocably This is known as subjective reality Heisenberg realised that ... In the world of very small particles, one cannot measure any property of a particle without interacting with it in some way This introduces an unavoidable uncertainty into the result One can never measure all the properties exactly Werner Heisenberg (1901-1976) Measuring the position and momentum of an electron Shine light on electron and detect reflected light using a microscope Minimum uncertainty in position is given by the wavelength of the light So to determine the position accurately, it is necessary to use light with a short wavelength Measuring the position and momentum of an electron (cont’d) By Planck’s law E = hc/l, a photon with a short wavelength has a large energy Thus, it would impart a large ‘kick’ to the electron But to determine its momentum accurately, electron must only be given a small kick This means using light of long wavelength! Fundamental Trade Off … Use light with short wavelength: – accurate measurement of position but not momentum Use light with long wavelength: – accurate measurement of momentum but not position Heisenberg’s Uncertainty Principle The more accurately you know the position (i.e., the smaller Dx is) , the less accurately you know the momentum (i.e., the larger Dp is); and vice versa applet Implications It is impossible to know both the position and momentum exactly, i.e., Dx=0 and Dp=0 These uncertainties are inherent in the physical world and have nothing to do with the skill of the observer Because h is so small, these uncertainties are not observable in normal everyday situations Example of Baseball A pitcher throws a 0.1-kg baseball at 40 m/s So momentum is 0.1 x 40 = 4 kg m/s Suppose the momentum is measured to an accuracy of 1 percent , i.e., Dp = 0.01 p = 4 x 10-2 kg m/s Example of Baseball (cont’d) The uncertainty in position is then No wonder one does not observe the effects of the uncertainty principle in everyday life! Example of Electron Same situation, but baseball replaced by an electron which has mass 9.11 x 10-31 kg So momentum = 3.6 x 10-29 kg m/s and its uncertainty = 3.6 x 10-31 kg m/s The uncertainty in position is then If Planck’s constant were much larger... Another Consequence of Heisenberg’s Uncertainty Principle A quantum particle can never be in a state of rest, as this would mean we know both its position and momentum precisely Thus, the carriage will be jiggling around the bottom of the valley forever Heisenberg’s Uncertainty Principle involving energy and time The more accurately we know the energy of a body, the less accurately we know how long it possessed that energy The energy can be known with perfect precision (DE = 0), only if the measurement is made over an infinite period of time (Dt = ∞) Summary: Lessons from Heisenberg The idea of a perfectly predictable universe cannot be true There is no such thing as an ideal, objective observer