Invisible tool enables new quantum experiments with atoms
... precisely measure tiny forces and displacements as well as to shed light onto the unexplored zone between the microscopic realm of quantum physics and our everyday world. Physicists around Philipp Haslinger and Markus Arndt at the University of Vienna have now succeeded in constructing a novel matte ...
... precisely measure tiny forces and displacements as well as to shed light onto the unexplored zone between the microscopic realm of quantum physics and our everyday world. Physicists around Philipp Haslinger and Markus Arndt at the University of Vienna have now succeeded in constructing a novel matte ...
Particle confined on a segment
... 11. Derive the expectation value of the position of the particle for a given n value. Comment. 12. Derive the expectation value of the momentum for a given n value. Comment. 13. We use the model of the confined particle along the segment [OL] to interpret the behavior of ! electrons of double bonds ...
... 11. Derive the expectation value of the position of the particle for a given n value. Comment. 12. Derive the expectation value of the momentum for a given n value. Comment. 13. We use the model of the confined particle along the segment [OL] to interpret the behavior of ! electrons of double bonds ...
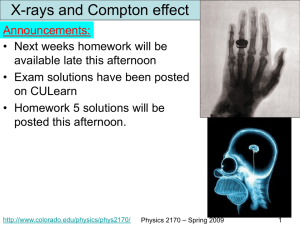
Physics 2170
... Blackbody radiation and the photoelectric effect can only be explained by a new quantum theory of light. The quantum of light is called a photon and it has energy of E = hf = hc/l. Furthermore, the Compton effect shows that photons carry momentum of p = h/l, consistent with the relativistic energymo ...
... Blackbody radiation and the photoelectric effect can only be explained by a new quantum theory of light. The quantum of light is called a photon and it has energy of E = hf = hc/l. Furthermore, the Compton effect shows that photons carry momentum of p = h/l, consistent with the relativistic energymo ...
Introduction: what is quantum field theory
... Another reason to treat the field, rather than the particle as the fundamental quantity is the experimental fact that when particles interact, new particles can be created (or annihilated) - the particle number is not conserved. This fact is demonstrated at a daily basis in CERN and other accelerato ...
... Another reason to treat the field, rather than the particle as the fundamental quantity is the experimental fact that when particles interact, new particles can be created (or annihilated) - the particle number is not conserved. This fact is demonstrated at a daily basis in CERN and other accelerato ...
Chapter 7
... Microscopic dynamics: Quantum mechanics • Classical physics failed to account for the existence of discrete energies of atoms and other experiments in the early 20th century. • Such total failures show that the basic concept of classical mechanics need to be corrected fundamentally. • A new mechani ...
... Microscopic dynamics: Quantum mechanics • Classical physics failed to account for the existence of discrete energies of atoms and other experiments in the early 20th century. • Such total failures show that the basic concept of classical mechanics need to be corrected fundamentally. • A new mechani ...
Physics 5002 (Spring 2017) Discussion Problem (4/20) Consider
... The energy of the particle is E = h̄2 k 2 /(2m) > V0 . Take the incident wave to be exp(ikx) in the region of x < 0. 1. Derive the amplitude A of the reflected wave A exp(−ikx) in the region of x < 0. 2. Derive the amplitude D of the transmitted wave D exp[ik(x − L)] in the region of x > L. 3. Show ...
... The energy of the particle is E = h̄2 k 2 /(2m) > V0 . Take the incident wave to be exp(ikx) in the region of x < 0. 1. Derive the amplitude A of the reflected wave A exp(−ikx) in the region of x < 0. 2. Derive the amplitude D of the transmitted wave D exp[ik(x − L)] in the region of x > L. 3. Show ...
Quantum Mechanics
... it doesn’t account for splitting of some spectral lines… it doesn’t account for interactions between atoms… and we haven’t explained “stationary states.” Looks like we’ve got some work to do. You may be on the right track, but… you’ll get run over if you just keep sitting there. ...
... it doesn’t account for splitting of some spectral lines… it doesn’t account for interactions between atoms… and we haven’t explained “stationary states.” Looks like we’ve got some work to do. You may be on the right track, but… you’ll get run over if you just keep sitting there. ...
in nm 1240 E in eV - Little Shop of Physics
... ow us only one face at a time. If we arrange an experiment to measure a ke property, such as interference, we find photons and electrons acting like not particles. An experiment to look for particles will find photons and eleccting like particles, not waves. These two aspects of light and matter are ...
... ow us only one face at a time. If we arrange an experiment to measure a ke property, such as interference, we find photons and electrons acting like not particles. An experiment to look for particles will find photons and eleccting like particles, not waves. These two aspects of light and matter are ...
Chapter 2 Study Guide
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
... 1. The first subatomic particle discovered was ___________________________. 2. The only subatomic particle that does not carry an electric charge is the __________. 3. The atomic number of an element whose atoms have 12 protons and 11 neutrons is _____. 4. The mass number of an element whose atoms h ...
Chapter 12 Worksheet
... b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the following list of observations, choose the one that most clearly ...
... b. neither the position nor the momentum can be measured precisely c. the position and the momentum of a particle can be measured precisely, but not at the same time d. the positon of a particle cannot be measured precisely 4. From the following list of observations, choose the one that most clearly ...
Quantum numbers
... finding the electron at position 1 relative to position 2. If the number is 100, the electron is 100 times more likely to be in position 1 than 2. ...
... finding the electron at position 1 relative to position 2. If the number is 100, the electron is 100 times more likely to be in position 1 than 2. ...
3.6 Wave particle duality
... The dual nature of light: The diffraction of light provides evidence of light being wavelike in nature The photo electric effect provides evidence of light being particle-like in nature The dual nature of matter: ...
... The dual nature of light: The diffraction of light provides evidence of light being wavelike in nature The photo electric effect provides evidence of light being particle-like in nature The dual nature of matter: ...
Quantum mechanics is the theory that we use to describe the
... objects angular momentum is due to its rotation around its central axis, or around an extended axis. Spin angular momentum in quantum mechanics does not arise from a particle actually spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is ...
... objects angular momentum is due to its rotation around its central axis, or around an extended axis. Spin angular momentum in quantum mechanics does not arise from a particle actually spinning like a top, rather it is an intrinsic property of a particle, like its mass. An important thing to note is ...