Force
... d. Describe how heat can be transferred through matter by the collisions of atoms (conduction) or through space (radiation). In a liquid or gas, currents will facilitate the transfer of ...
... d. Describe how heat can be transferred through matter by the collisions of atoms (conduction) or through space (radiation). In a liquid or gas, currents will facilitate the transfer of ...
QUANTUM NUMBERS
... this (1897) this was explained by Sommerfeld & Debye (1916) who thought that orbits may exist at varying angles and that the energies may be different when near strong magnets for each value of l, ml can vary from –l to +l (each value represents a different orientation) i.e. if l=1 then ml can ...
... this (1897) this was explained by Sommerfeld & Debye (1916) who thought that orbits may exist at varying angles and that the energies may be different when near strong magnets for each value of l, ml can vary from –l to +l (each value represents a different orientation) i.e. if l=1 then ml can ...
The Bohr model for the electrons
... Couldn’t explain why orbits were allowed Only successful agreement with experiment was with the H atom Introduced connection between spectra and electron structure Concept of allowed orbits is developed further with new knowledge Nonetheless, an important contribution, worthy of the Nobel prize ...
... Couldn’t explain why orbits were allowed Only successful agreement with experiment was with the H atom Introduced connection between spectra and electron structure Concept of allowed orbits is developed further with new knowledge Nonetheless, an important contribution, worthy of the Nobel prize ...
Example Chapter Outline – Chemistry
... Alpha particle: helium nucleus 42He Alpha particle production: very common mode of decay for heavy radioactive nuclides Beta particle production: another common decay process Gamma ray: a high-energy photon of light Positron: a particle with the same mass as electron by opposite charge Positron prod ...
... Alpha particle: helium nucleus 42He Alpha particle production: very common mode of decay for heavy radioactive nuclides Beta particle production: another common decay process Gamma ray: a high-energy photon of light Positron: a particle with the same mass as electron by opposite charge Positron prod ...
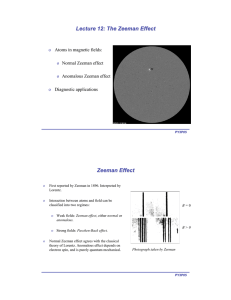
o Atoms in magnetic fields: Normal Zeeman effect Anomalous Zeeman effect
... Consider transitions between two Zeeman-split atomic levels. Allowed transition frequencies are therefore, ...
... Consider transitions between two Zeeman-split atomic levels. Allowed transition frequencies are therefore, ...
Serge Haroche
... Particle control in a quantum world Serge Haroche and David J. Wineland have independently invented and developed methods for measuring and manipulating individual particles while preserving their quantum-mechanical nature, in ways that were previously thought unattainable. The Nobel Laureates have ...
... Particle control in a quantum world Serge Haroche and David J. Wineland have independently invented and developed methods for measuring and manipulating individual particles while preserving their quantum-mechanical nature, in ways that were previously thought unattainable. The Nobel Laureates have ...
Assumed Knowledge and Skills
... The Stage 2 Physics subject outline assumes that students are familiar with the concepts listed below. These concepts are grouped under the section of the Subject Outline in which they are first needed. The Physics Investigation Skills section and the Content section of the subject outline indicate ...
... The Stage 2 Physics subject outline assumes that students are familiar with the concepts listed below. These concepts are grouped under the section of the Subject Outline in which they are first needed. The Physics Investigation Skills section and the Content section of the subject outline indicate ...
Photon Frequency Shift Caused by Gravity and Its Electromagnetic
... It has also been reported that atoms absorb these TEM waves in the frequency range 0.005 - 0.03 Hz [1]. The absorbed energy is very large and may account for atomic forces and atomic stored energy. Furthermore, it has been reported that atoms also absorb TEM waves with frequency 69.9 Hz [2]. These T ...
... It has also been reported that atoms absorb these TEM waves in the frequency range 0.005 - 0.03 Hz [1]. The absorbed energy is very large and may account for atomic forces and atomic stored energy. Furthermore, it has been reported that atoms also absorb TEM waves with frequency 69.9 Hz [2]. These T ...
Enrichment Opportunities: Atoms
... The never-ending quest to better understand our universe has led scientists to some amazing places, from the deep reaches of outer space to the heart of the atom. Our curiosity has shown us things smaller than anyone thought existed – first the atom and then subatomic particles. But scientists didn’ ...
... The never-ending quest to better understand our universe has led scientists to some amazing places, from the deep reaches of outer space to the heart of the atom. Our curiosity has shown us things smaller than anyone thought existed – first the atom and then subatomic particles. But scientists didn’ ...
Quantum and Atomic Physics - Problems PSI AP Physics 2
... 23. Who demonstrated the existence of the neutron? What role does the neutron play in the nucleus? 24. Which physicists described light as a particle? Which physicists described light as a wave? 25. Who was the first to propose that particles (such as electrons) could act as waves? Why do we not see ...
... 23. Who demonstrated the existence of the neutron? What role does the neutron play in the nucleus? 24. Which physicists described light as a particle? Which physicists described light as a wave? 25. Who was the first to propose that particles (such as electrons) could act as waves? Why do we not see ...