Chapter 4 Test Question Topics
... Chapter 4 Test Question Topics 20 multiple choice questions. 1- Know the definitions of the ground state and the excited states of an atom. 2- What must occur for an atom to move from the ground to the excited state or from the excited to the ground state? 3- Know the definitions of an electron clo ...
... Chapter 4 Test Question Topics 20 multiple choice questions. 1- Know the definitions of the ground state and the excited states of an atom. 2- What must occur for an atom to move from the ground to the excited state or from the excited to the ground state? 3- Know the definitions of an electron clo ...
Physics 2170
... spin in every direction but experiments can only get the limited knowledge allowed by quantum mechanics. A better theory would allow one to get access to this information. This is called a hidden variable theory. In 1964, J.S Bell proved that local hidden variable theories would give a different res ...
... spin in every direction but experiments can only get the limited knowledge allowed by quantum mechanics. A better theory would allow one to get access to this information. This is called a hidden variable theory. In 1964, J.S Bell proved that local hidden variable theories would give a different res ...
Problem-Solving Strategy
... where is the angle between the incident polarization direction and the filter’s polarizing axis. ...
... where is the angle between the incident polarization direction and the filter’s polarizing axis. ...
Δk/k
... if rate of change of Hamiltonian << Bohr frequency. Non-adiabatic change of Hamiltonian: System stays as it is. B NMR: Adiabatic fast passage. σ Ωt = π t ...
... if rate of change of Hamiltonian << Bohr frequency. Non-adiabatic change of Hamiltonian: System stays as it is. B NMR: Adiabatic fast passage. σ Ωt = π t ...
ppt - Physics
... chemical changes in an irradiated material is the energy absorbed from the radiation field. Dosimetry provides a way to determine the amount of energy that has been absorbed by the irradiated material from the radiation. The dose D, is the amount of energy absorbed per unit mass of material. ...
... chemical changes in an irradiated material is the energy absorbed from the radiation field. Dosimetry provides a way to determine the amount of energy that has been absorbed by the irradiated material from the radiation. The dose D, is the amount of energy absorbed per unit mass of material. ...
Lecture 12
... and momentum. The second term on the left side, Mo dt, is called the angular impulse. In cases of 2D motion, it can be applied as a scalar equation using components about the z-axis. ...
... and momentum. The second term on the left side, Mo dt, is called the angular impulse. In cases of 2D motion, it can be applied as a scalar equation using components about the z-axis. ...
Atomic Theory - Buford High School Chemistry
... Most of the alpha particles passed through the gold foil ______________. A smaller percentage of the particles were slightly _________________. A very small number of particles were deflected _____________ ________ towards their source. From these observations he concluded the following: The ...
... Most of the alpha particles passed through the gold foil ______________. A smaller percentage of the particles were slightly _________________. A very small number of particles were deflected _____________ ________ towards their source. From these observations he concluded the following: The ...
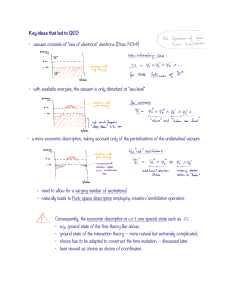
Key ideas that led to QED vacuum consists of "sea of electrons
... Only then, the n-th sum and of the above expression makes sense. Even after all these manipulations, convergence of the sequence is unknown. • Not every Taylor series has a sufficiently large convergence radius • A simple example that caricatures the problem is the following: ...
... Only then, the n-th sum and of the above expression makes sense. Even after all these manipulations, convergence of the sequence is unknown. • Not every Taylor series has a sufficiently large convergence radius • A simple example that caricatures the problem is the following: ...
2003 The McGraw-Hill Companies, Inc. All rights reserved. 14
... can be applied to relate the initial kinetic energy to the maximum potential energy. The maximum vertical distance is determined from this relation. ...
... can be applied to relate the initial kinetic energy to the maximum potential energy. The maximum vertical distance is determined from this relation. ...
Name________________________________ Block ______ Date
... A is the ending amount, C is starting amount, r is the rate of increase or decrease as a decimal(divide % by 100) Practice B – Use the growth or decay formula to find the new amounts 1. The average rent in Mecklenburg county in 2011 was $858. The next year it was expected to go up by 10.5%. What wou ...
... A is the ending amount, C is starting amount, r is the rate of increase or decrease as a decimal(divide % by 100) Practice B – Use the growth or decay formula to find the new amounts 1. The average rent in Mecklenburg county in 2011 was $858. The next year it was expected to go up by 10.5%. What wou ...