- Biglobe
... only include effect by electric field “RE” although the derived interaction equation for a charged particle include effects by both electric field (Coulomb force) and magnetic field (Lorentz force). The reason is that we assume the motion of a charged particle as r r0 eit r0*e it which is vi ...
... only include effect by electric field “RE” although the derived interaction equation for a charged particle include effects by both electric field (Coulomb force) and magnetic field (Lorentz force). The reason is that we assume the motion of a charged particle as r r0 eit r0*e it which is vi ...
principles1 - UCL Department of Geography
... •radiation emitted from unit area of any plane surface with emissivity of (<1) can be written • = Tn where n is a numerical index •For ‘grey’ surface where is nearly independent of, n =4 •When radiation emitted predominantly at < m , n > 4 • When radiation emitted predominantly at > ...
... •radiation emitted from unit area of any plane surface with emissivity of (<1) can be written • = Tn where n is a numerical index •For ‘grey’ surface where is nearly independent of, n =4 •When radiation emitted predominantly at < m , n > 4 • When radiation emitted predominantly at > ...
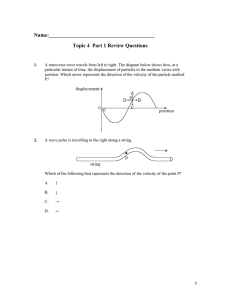
What do you remember from Physics 53?
... finger. If the head of hammer is heavy and the handle long, would it be easier to balance with the end of the handle on your finger so that the head is at the top, or the other way around with the head at your fingertip and the end of the handle on the top? 2. Consider a pair of meter sticks standin ...
... finger. If the head of hammer is heavy and the handle long, would it be easier to balance with the end of the handle on your finger so that the head is at the top, or the other way around with the head at your fingertip and the end of the handle on the top? 2. Consider a pair of meter sticks standin ...
PHYS4210 Electromagnetic Theory Spring 2009 Final Exam
... where e and m are the electron charge and mass, and N is the electron density. If ω corresponds to the angular frequency of visible light, then ω0 would be given by the angular frequency of electromagnetic radiation A. in the microwave region. B. in the infrared region. C. in the green region. D. in ...
... where e and m are the electron charge and mass, and N is the electron density. If ω corresponds to the angular frequency of visible light, then ω0 would be given by the angular frequency of electromagnetic radiation A. in the microwave region. B. in the infrared region. C. in the green region. D. in ...
EP-307 Introduction to Quantum Mechanics
... Does It mean that 50% of the atoms in the Sz+ beam coming out Of the first apparatus are made of atoms characterized by Sx+ & 50% of the time by Sx- ...
... Does It mean that 50% of the atoms in the Sz+ beam coming out Of the first apparatus are made of atoms characterized by Sx+ & 50% of the time by Sx- ...
Physics 9 Fall 2009 - faculty.ucmerced.edu
... where E0 is the amplitude, k = 2π/λ is the wave number, and ω = 2πf is the angular frequency. So, everything is defined as before. (a) The wavelength λ = ...
... where E0 is the amplitude, k = 2π/λ is the wave number, and ω = 2πf is the angular frequency. So, everything is defined as before. (a) The wavelength λ = ...
Chapter 34
... frequency of the receiver was adjusted to match that of the transmitter. In a series of other experiments, Hertz also showed that the radiation generated by this equipment exhibited wave properties. Interference, diffraction, reflection, refraction and polarization He also measured the speed of th ...
... frequency of the receiver was adjusted to match that of the transmitter. In a series of other experiments, Hertz also showed that the radiation generated by this equipment exhibited wave properties. Interference, diffraction, reflection, refraction and polarization He also measured the speed of th ...
1 - contentextra
... are bombarded with electrons of high kinetic energy which ejects an electron from an outer energy (valence) level. Both electrons leave the atom: X(g) + e– → X–(g) +2e–. Line spectrum A line spectrum is an emission spectrum with only certain frequencies. It is produced by excited atoms and ions as ...
... are bombarded with electrons of high kinetic energy which ejects an electron from an outer energy (valence) level. Both electrons leave the atom: X(g) + e– → X–(g) +2e–. Line spectrum A line spectrum is an emission spectrum with only certain frequencies. It is produced by excited atoms and ions as ...
Principle of Impulse and momentum
... The smooth disks A and B, having a mass of 1 kg and 2 kg, respectively, collide with the velocities shown in the figure. If e = 0.75, determine the x and y components of the final velocity of each disk just after collision. ...
... The smooth disks A and B, having a mass of 1 kg and 2 kg, respectively, collide with the velocities shown in the figure. If e = 0.75, determine the x and y components of the final velocity of each disk just after collision. ...
Atomic Structure and Chemical Bonding
... Ans2. Lyman series: When excited electrons in hydrogen atoms fall from higher energy levels to first energy level, the series of lines observed are called Lyman series. They are observed in ultraviolet region. Lyman = R(1/12 - 1/n2), n = 2, 3, 4, 5, ......... Balmer series: When excited electrons in ...
... Ans2. Lyman series: When excited electrons in hydrogen atoms fall from higher energy levels to first energy level, the series of lines observed are called Lyman series. They are observed in ultraviolet region. Lyman = R(1/12 - 1/n2), n = 2, 3, 4, 5, ......... Balmer series: When excited electrons in ...