Chapter 7 The Quantum-Mechanical Model of the Atom
... Angular Momentum Quantum Number - primarily determines the shape of the orbital - can have values of any integer from l = 0 …. (n – 1) - each value of l is called by a particular letter that designates the shape of the orbital i.) if l=0, called s orbitals and are spherical. ii.) if l=1, called p or ...
... Angular Momentum Quantum Number - primarily determines the shape of the orbital - can have values of any integer from l = 0 …. (n – 1) - each value of l is called by a particular letter that designates the shape of the orbital i.) if l=0, called s orbitals and are spherical. ii.) if l=1, called p or ...
Complex Numbers Worksheet
... 1) At an amusement park a ride called the rotor is a cylindrical room that spins around. The riders stand against the circular wall. When the rotor reaches the necessary speed, the floor drops out and the centrifugal force keeps the riders pinned to the wall. The model that gives the speed s (in met ...
... 1) At an amusement park a ride called the rotor is a cylindrical room that spins around. The riders stand against the circular wall. When the rotor reaches the necessary speed, the floor drops out and the centrifugal force keeps the riders pinned to the wall. The model that gives the speed s (in met ...
Slide 101
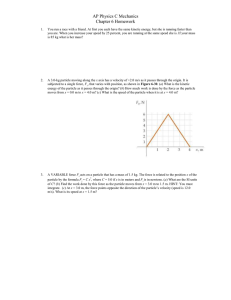
... (a) Normalize the wave function. (b) Find the probability that the particle will be found in the region 0 x L / 4. 2. No optical instrument can resolve the structural details of an object smaller than the wavelength of light by which it is being observed. For this reason, although an optical mi ...
... (a) Normalize the wave function. (b) Find the probability that the particle will be found in the region 0 x L / 4. 2. No optical instrument can resolve the structural details of an object smaller than the wavelength of light by which it is being observed. For this reason, although an optical mi ...
Review
... plastic bag while holding it in your hands. Would you conclude from this that glass is a conductor or an insulator? Why? 2. Why it is easier to charge a balloon on a dry day than on a humid day? ...
... plastic bag while holding it in your hands. Would you conclude from this that glass is a conductor or an insulator? Why? 2. Why it is easier to charge a balloon on a dry day than on a humid day? ...
How close can we get waves to wavefunctions, including potential?
... Δx is the distance between the beads. Since the right-hand side of equation (3) represents mass times acceleration, all terms on the left-hand side are obviously forces. The second term is thus a force proportional to displacement. This can be a spring obeying Hooke's law and K(x) is then the spring ...
... Δx is the distance between the beads. Since the right-hand side of equation (3) represents mass times acceleration, all terms on the left-hand side are obviously forces. The second term is thus a force proportional to displacement. This can be a spring obeying Hooke's law and K(x) is then the spring ...
Jeopardy Review
... To produce more electrons per unit time but with less kinetic energy per electron, the experimenter should do which of the following? (A) Increase the intensity and decrease the wavelength of the light. (B) Increase the intensity and the wavelength of the light. (C) Decrease the intensity and the wa ...
... To produce more electrons per unit time but with less kinetic energy per electron, the experimenter should do which of the following? (A) Increase the intensity and decrease the wavelength of the light. (B) Increase the intensity and the wavelength of the light. (C) Decrease the intensity and the wa ...
Principles of Scientific Simulation
... • Quantum effects are only important at small distances (10-13 cms for the so called strong or nuclear forces, 10-8 cm for electromagnetically interacting particles). • Often these short distance effects are unimportant and it is sufficient to treat physics classically. Then all matter is made up of ...
... • Quantum effects are only important at small distances (10-13 cms for the so called strong or nuclear forces, 10-8 cm for electromagnetically interacting particles). • Often these short distance effects are unimportant and it is sufficient to treat physics classically. Then all matter is made up of ...
Atomic Physics
... The Bohr Theory is very successful in predicting the energy levels of the hydrogen atom. It also suggests that the electron has a wave-like nature as described above. However, it does not completely describe other quantum effects and it does not sufficiently describe the wave nature of the electron. ...
... The Bohr Theory is very successful in predicting the energy levels of the hydrogen atom. It also suggests that the electron has a wave-like nature as described above. However, it does not completely describe other quantum effects and it does not sufficiently describe the wave nature of the electron. ...
Waves, part 9 - UCSD Department of Physics
... EM Waves by an Antenna • Because the oscillating charges in the rod produce a current, there is also a magnetic field generated • As the current changes, the magnetic field spreads out from the ...
... EM Waves by an Antenna • Because the oscillating charges in the rod produce a current, there is also a magnetic field generated • As the current changes, the magnetic field spreads out from the ...
History and the State-of-art in Quantum Computation
... History and the State-of-art in Quantum Computation ...
... History and the State-of-art in Quantum Computation ...
Smallest sliver of time yet measured sees electrons
... In a series of experiments, the team fired an unspeakably brief, extremely ultraviolet laser pulse at a helium atom to start exciting its pair of electrons. This pulse lasted just 100 to 200 attoseconds, or 10-18 seconds. But by making many readings and calculating their statistical spread, they wer ...
... In a series of experiments, the team fired an unspeakably brief, extremely ultraviolet laser pulse at a helium atom to start exciting its pair of electrons. This pulse lasted just 100 to 200 attoseconds, or 10-18 seconds. But by making many readings and calculating their statistical spread, they wer ...