Modern physics 2330
... 4- ( ) The number, strength, and exact position of the lines in the spectrum of an element depend only upon temperature. 5- ( ) According to de Broglie, the electron of the Bohr atom forms a standing h wave around the nucleus with . p 6- ( ) Davisson-Germer experiment (1927) is a direct experime ...
... 4- ( ) The number, strength, and exact position of the lines in the spectrum of an element depend only upon temperature. 5- ( ) According to de Broglie, the electron of the Bohr atom forms a standing h wave around the nucleus with . p 6- ( ) Davisson-Germer experiment (1927) is a direct experime ...
Homework 3
... 1. Explain the terms wavelength and amplitude. If a photon has a frequency of 9 1010 Hz what is its wavelength? Which region of the electromagnetic spectrum does this correspond to? ...
... 1. Explain the terms wavelength and amplitude. If a photon has a frequency of 9 1010 Hz what is its wavelength? Which region of the electromagnetic spectrum does this correspond to? ...
Modern Physics Lesson 3
... Conclusion: A photon is a particle of light that has energy and momentum. However, photons have no mass and travel at the speed of light, c. deBroglie Wavelength Louis deBroglie proposed in 1923 that if waves behave like particles, then particles should also behave like waves! This was the beginnin ...
... Conclusion: A photon is a particle of light that has energy and momentum. However, photons have no mass and travel at the speed of light, c. deBroglie Wavelength Louis deBroglie proposed in 1923 that if waves behave like particles, then particles should also behave like waves! This was the beginnin ...
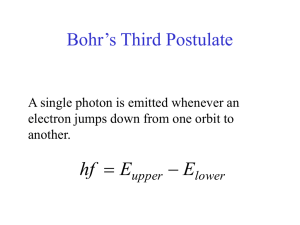
Bohr´s Third Postulate
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
... don’t all the photoelectrons have the same kinetic energy when they leave the metal’s surface? 4. What property of the emitted electrons depends on the intensity of incident light?What property of the emitted photoelectrons depends on the frequency of incident light? ...
lect10
... an atom has a number of stable states in which the electrons orbit the nucleus in accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only ...
... an atom has a number of stable states in which the electrons orbit the nucleus in accordance with Newton’s Laws but do not radiate energy an atom emits or absorbs energy only ...