Lecture 1
... particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles, such as electrons or protons, have properties characteristic of both waves ...
... particles. The need for a revision of the foundations of mechanics arises as a result of the wave-particle duality of matter, which manifests itself in systems of atomic dimensions. Wave-particle duality means that particles, such as electrons or protons, have properties characteristic of both waves ...
$doc.title
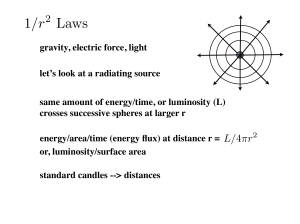
... Summary: Classical concepts: Particle mechanics, Electromagnetism, Waves, Diffraction, Relativity, Thermodynamics ...
... Summary: Classical concepts: Particle mechanics, Electromagnetism, Waves, Diffraction, Relativity, Thermodynamics ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI
... 16) (i) Explain how the laws of conservation of relativistic energy and momentum establish the observed change in wavelength of X-ray ray in Compton scattering (ii) Establish the work-energy energy theorem of relativity. relativity ...
... 16) (i) Explain how the laws of conservation of relativistic energy and momentum establish the observed change in wavelength of X-ray ray in Compton scattering (ii) Establish the work-energy energy theorem of relativity. relativity ...
T3_Static_Potentials_And_Eigenstates
... – Found by accident (“faulty” nickel target) – “ruined” Germer’s second honeymoon ...
... – Found by accident (“faulty” nickel target) – “ruined” Germer’s second honeymoon ...
Student Presentation
... – Energy is “quantized” meaning it can only exist at certain energy levels and not in between • Like rungs on a ladder ...
... – Energy is “quantized” meaning it can only exist at certain energy levels and not in between • Like rungs on a ladder ...
Problem set 3
... 1. Recall that the angular momentum raising operator is L+ = ~eiφ (∂θ + i cot θ ∂φ ). Use this to find L− . 2. Use the above formulae for L± to find the coordinate representation of the angular momentum basis states Y11 , Y10 and Y1,−1 up to normalization. 3. Write out the 9 equations summarized in ...
... 1. Recall that the angular momentum raising operator is L+ = ~eiφ (∂θ + i cot θ ∂φ ). Use this to find L− . 2. Use the above formulae for L± to find the coordinate representation of the angular momentum basis states Y11 , Y10 and Y1,−1 up to normalization. 3. Write out the 9 equations summarized in ...
Consider the following solution to the hydrogen atom problem
... b) What is the angular momentum quantum number, l associated with this state? c) What is the expectation of the angular momentum projection? In other words, < Lz> = ? d) What is the probability of finding the electron somewhere along the z-axis? e) What is the probability of finding the electron at ...
... b) What is the angular momentum quantum number, l associated with this state? c) What is the expectation of the angular momentum projection? In other words, < Lz> = ? d) What is the probability of finding the electron somewhere along the z-axis? e) What is the probability of finding the electron at ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI-600034 M.Sc. Part-A NOVEMBER 2015
... dimensionality of the irreducible representations. What are coulomb and exchange integrals? How are they obtained? ...
... dimensionality of the irreducible representations. What are coulomb and exchange integrals? How are they obtained? ...
Adobe Acrobat file ()
... cannot have the same speed because of the difference in their masses. For the same reason, remembering that KE = p2/2m, they cannot have the same kinetic energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different ...
... cannot have the same speed because of the difference in their masses. For the same reason, remembering that KE = p2/2m, they cannot have the same kinetic energy. Because the kinetic energy is the only type of energy an isolated particle can have, and we have argued that the particles have different ...
UNIVERSITY OF LEIPZIG
... 26. A transverse electromagnetic wave (E and H components are perpendicular to the propagation direction and perpendicular to each other) is incident normally in vacuum on a perfectly absorbing flat screen. ...
... 26. A transverse electromagnetic wave (E and H components are perpendicular to the propagation direction and perpendicular to each other) is incident normally in vacuum on a perfectly absorbing flat screen. ...
A system consist of two particles,each of which has two possible
... with energies Eo and 2Eo .Write the complete expression for the partition function if: (a) The particle are distinguishable. (b) The particle obey Maxwell-Boltzmann statistics. (c) The particle obey Fermi-Dirac statistics. (d) The particle obey Bose-Einstein statistics. 2.When a closed cubic box of ...
... with energies Eo and 2Eo .Write the complete expression for the partition function if: (a) The particle are distinguishable. (b) The particle obey Maxwell-Boltzmann statistics. (c) The particle obey Fermi-Dirac statistics. (d) The particle obey Bose-Einstein statistics. 2.When a closed cubic box of ...
File
... B) behave like waves, but are particles C) behave like particles, but are waves. D) are both waves and particles. b. This shows the electrons have a dual nature, physicists call this nature ____________ - _______________ __________________. 7. Since it behaves like a wave, an electron has a waveleng ...
... B) behave like waves, but are particles C) behave like particles, but are waves. D) are both waves and particles. b. This shows the electrons have a dual nature, physicists call this nature ____________ - _______________ __________________. 7. Since it behaves like a wave, an electron has a waveleng ...