Chapter 5 PPT/Notes A
... • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. • Frequency is how many waves per unit of time pass a point. • Period is how long it takes for a wavelength to pass a point. • F=1/period ...
... • The trough is the ‘bottom-point’ of a wave and the crest is the ‘peak’ of a wave. • Frequency is how many waves per unit of time pass a point. • Period is how long it takes for a wavelength to pass a point. • F=1/period ...
Physical Chemistry II Review Set 1
... a. Explain black body radiation and why it is so important. Sketch a black body curve. Label the axes. b. Explain the significance of the double slit experiment for electrons c. Explain the significance of the photoelectric effect. 6. A laser emits photons with a wavelength of 1064 nm with a power o ...
... a. Explain black body radiation and why it is so important. Sketch a black body curve. Label the axes. b. Explain the significance of the double slit experiment for electrons c. Explain the significance of the photoelectric effect. 6. A laser emits photons with a wavelength of 1064 nm with a power o ...
3.4oquantum.4u
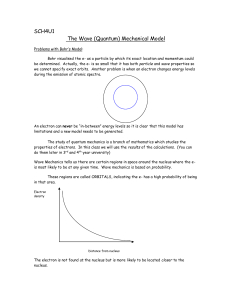
... Problems with Bohr’s Model: Bohr visualised the e- as a particle by which its exact location and momentum could be determined. Actually, the e- is so small that it has both particle and wave properties so we cannot specify exact orbits. Another problem is when an electron changes energy levels durin ...
... Problems with Bohr’s Model: Bohr visualised the e- as a particle by which its exact location and momentum could be determined. Actually, the e- is so small that it has both particle and wave properties so we cannot specify exact orbits. Another problem is when an electron changes energy levels durin ...
Notes27and29January2014BasicQuantumMechanics
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
... Quantum Theory for Semiconductors How to determine the behavior of electrons in the semiconductor? • Mathematical description of motion of electrons in quantum mechanics ─ Schrödinger’s Equation • Solution of Schrödinger’s Equation energy band structure and probability of finding a electron at a pa ...
MIT Physics Graduate General Exams
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
... Students studying for the Fall 2003 Part I exam found it useful to first categorize each problem by the concept they thought was being tested. Such an approach simplified their subsequent attempt at solving the problem. The following is a list of all the concepts they thought could be used as the ba ...
Quantum Mechanical Energy and You!
... In the new realm of thought, macroscopic objects now be thought of in terms of their matter waves or Debroglie waves. Waves are quantities that are highly non local and spread through space in time. Thus, the idea of how we talk of the fundamental quantity position must be re-invented, as well as al ...
... In the new realm of thought, macroscopic objects now be thought of in terms of their matter waves or Debroglie waves. Waves are quantities that are highly non local and spread through space in time. Thus, the idea of how we talk of the fundamental quantity position must be re-invented, as well as al ...
BasicQuantumMechanics18And20January2017
... • It is impossible to simultaneously describe with absolute accuracy the energy of a particle and the instant of time the particle has this energy ...
... • It is impossible to simultaneously describe with absolute accuracy the energy of a particle and the instant of time the particle has this energy ...
Pre-AP Chemistry
... Radiation and Spectra Review. Complete the following short answer questions. 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What ...
... Radiation and Spectra Review. Complete the following short answer questions. 1. What kind of proportion is shown by the equation c = λ ν? 2. Who stated that electromagnetic radiation has a dual nature? 3. How many meters are 665 nm equal to? 4. What wave measure is identified by the unit Hz? 5. What ...
Quantum Mechanics
... light was a wave (Newton thought it was a particle, but Young disproved his theories) According to wave theory, it should not depend on color of light, but rather the intensity Einstein determined that the photoelectric effect is in fact proof that light is a photon (particle) and has energy bas ...
... light was a wave (Newton thought it was a particle, but Young disproved his theories) According to wave theory, it should not depend on color of light, but rather the intensity Einstein determined that the photoelectric effect is in fact proof that light is a photon (particle) and has energy bas ...
Quantum_PPT
... frequency (f0)ejects electrons no matter what intensity the light. • B) Photon- electromagnetic radiation travels in packets with a given amount of energy based on the frequency of the radiation. [Einstein] • E = hf ...
... frequency (f0)ejects electrons no matter what intensity the light. • B) Photon- electromagnetic radiation travels in packets with a given amount of energy based on the frequency of the radiation. [Einstein] • E = hf ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B 6. What are unitary transformations? 7. Show that commuting operators have simultaneous eigenfunctions. 8. What are indistinguishable particles? 9. What is ...
... 4. Prove that the square of the angular momentum commutes with its z-component. 5. If A and B are two operators, then show that [A-1[A,B]] = 2B 6. What are unitary transformations? 7. Show that commuting operators have simultaneous eigenfunctions. 8. What are indistinguishable particles? 9. What is ...
ph 2811 / 2808 - quantum mechanics
... 9. What are symmetric and antisymmetric wave functions? Show that the symmetry character of a wave function does not change with time. Explain how symmetric and antisymmetric wave functions are constructed from unsymmetrized solution of the schrodinger equation of a system of indistinguishable parti ...
... 9. What are symmetric and antisymmetric wave functions? Show that the symmetry character of a wave function does not change with time. Explain how symmetric and antisymmetric wave functions are constructed from unsymmetrized solution of the schrodinger equation of a system of indistinguishable parti ...
Chemistry 1 Concept 5 “Electrons in Atoms” Study Guide
... current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, d ______, f ______ 5. What is the wavelength of microwaves of 4.0 x 109 Hz frequency? ...
... current is passed through a tube of hydrogen gas at low pressure is _________________ 3. The energy of a photon is related to its ______________ 4. Give the number of orbitals for each sublevel: s _____, p ______, d ______, f ______ 5. What is the wavelength of microwaves of 4.0 x 109 Hz frequency? ...
Atomic Diffraction Dr. Janine Shertzer College of the Holy Cross
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
... The wave-particle duality is fundamental to quantum mechanics. Light can behave like a particle (photon); matter can behave like a wave. The wavelength associated with a particle is inversely proportional to its momentum p: λ = h / p, where h is Planck’s constant. For cold atoms, the wavelength is l ...
Development of Quantum Mechanics Waves
... If light is a wave with particle properties, are electrons particles with wave properties? C. J. Davisson and L. H. Germier at Bell labs proved that electrons produce diffraction patterns and verified deBroglies hypothesis ...
... If light is a wave with particle properties, are electrons particles with wave properties? C. J. Davisson and L. H. Germier at Bell labs proved that electrons produce diffraction patterns and verified deBroglies hypothesis ...
2710 PS3 1 Problem Set #3 Comparing classical electromagnetic
... Problem 1 refers to: A standing electric field wave (one with lots of photons) in a quantum wire stretching between x = 0 and x = L is described by E(x,t)=Emax sin(3π x L)cos(3π ct L) . Let L = 900 nm. 1.(a) What is the wavelength of the wave? What is the ordinary frequency? In which part of the ele ...
... Problem 1 refers to: A standing electric field wave (one with lots of photons) in a quantum wire stretching between x = 0 and x = L is described by E(x,t)=Emax sin(3π x L)cos(3π ct L) . Let L = 900 nm. 1.(a) What is the wavelength of the wave? What is the ordinary frequency? In which part of the ele ...
Homework 2
... (b) According to the Bohr model, electrons move on circular orbits and the angular momentum L can assume the values L = n~, n ∈ {1, 2, . . .}. Determine the possible energies En , orbital radii rn , and velocity ratios vn /c, where c is the speed of light. (c) Bohr assumed that only radiation of fre ...
... (b) According to the Bohr model, electrons move on circular orbits and the angular momentum L can assume the values L = n~, n ∈ {1, 2, . . .}. Determine the possible energies En , orbital radii rn , and velocity ratios vn /c, where c is the speed of light. (c) Bohr assumed that only radiation of fre ...