Quantum Mechanics
... Features of phase space trajectories (these are not particle coordinate trajectories in time) 1) Constants of motion do not change along a phase space trajectory (PST) 2) Isolated system moves in a small part of PST along which the constants of motion are fixed 3) Ergodic hypothesis: A trajectory p ...
... Features of phase space trajectories (these are not particle coordinate trajectories in time) 1) Constants of motion do not change along a phase space trajectory (PST) 2) Isolated system moves in a small part of PST along which the constants of motion are fixed 3) Ergodic hypothesis: A trajectory p ...
Exam 2 Review - Iowa State University
... 1060 Hixson-Lied Student Success Center 515-294-6624 [email protected] http://www.si.iastate.edu ...
... 1060 Hixson-Lied Student Success Center 515-294-6624 [email protected] http://www.si.iastate.edu ...
Quantum Mechanics
... and momentum but by something called a "wavefunction." knowing the state of a particle (i.e., its wavefunction) does not enable you to predict the results of measurements with certainty, but rather gives you a set of probabilities for the possible outcomes of any measurement. The wave-function is ...
... and momentum but by something called a "wavefunction." knowing the state of a particle (i.e., its wavefunction) does not enable you to predict the results of measurements with certainty, but rather gives you a set of probabilities for the possible outcomes of any measurement. The wave-function is ...
SCHRÖDINGER EQUATION FOR A PARTICLE ON A CURVED SPACE AND SUPERINTEGRABILITY
... determined, one for each case. It is shown how the Schrödinger equation can be rendered separable in each of the cases. ...
... determined, one for each case. It is shown how the Schrödinger equation can be rendered separable in each of the cases. ...
Chapter 27 - Planet Holloway
... By applying a voltage between the surface and the tip, the electrons can be made to tunnel preferentially from surface to tip The tip samples the distribution of electrons just above the surface The STM is very sensitive to the distance between the surface and the ...
... By applying a voltage between the surface and the tip, the electrons can be made to tunnel preferentially from surface to tip The tip samples the distribution of electrons just above the surface The STM is very sensitive to the distance between the surface and the ...
chapter27
... By applying a voltage between the surface and the tip, the electrons can be made to tunnel preferentially from surface to tip The tip samples the distribution of electrons just above the surface The STM is very sensitive to the distance between the surface and the ...
... By applying a voltage between the surface and the tip, the electrons can be made to tunnel preferentially from surface to tip The tip samples the distribution of electrons just above the surface The STM is very sensitive to the distance between the surface and the ...
107 chem Assement Q
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
... c. fundamental state. d. original state. 5. The hydrogen emission spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. the n = 3 state to the n = 2 state. b. the n = 4 state to the n = 2 state. c. the n = 5 state to the n = 2 state. d. the n = 6 ...
You are going to read the chapter at home.
... Fermions are easier than bosons because the occupation numbers are more limited: for any single-particle state i, ni can only be 0 or 1. That’s the Pauli exclusion principle. It is a consequence of the antisymmetry of the wave function. ...
... Fermions are easier than bosons because the occupation numbers are more limited: for any single-particle state i, ni can only be 0 or 1. That’s the Pauli exclusion principle. It is a consequence of the antisymmetry of the wave function. ...
Classical: electron as particle
... Lise Meitner was part of the team that discovered nuclear fission, an achievement for which her colleague Otto Hahn was awarded the Nobel Prize. Meitner is often mentioned as one of the most glaring examples of scientific achievement overlooked by the Nobel committee.[2][3][4] A 1997 Physics Today s ...
... Lise Meitner was part of the team that discovered nuclear fission, an achievement for which her colleague Otto Hahn was awarded the Nobel Prize. Meitner is often mentioned as one of the most glaring examples of scientific achievement overlooked by the Nobel committee.[2][3][4] A 1997 Physics Today s ...
Intro to EMR and Wave Equation
... • The electric field is parallel to the conductor and the magnetic field is perpendicular to the conductor ...
... • The electric field is parallel to the conductor and the magnetic field is perpendicular to the conductor ...
2-slit experiments with bullets (classical particles)
... • Wait--we can slow gun down so that only 1 electron per hour goes through. Then we expect electron goes through slit 1 or 2, right? Every hour we get a new spot on the screen. • Interference pattern builds up slowly: ...
... • Wait--we can slow gun down so that only 1 electron per hour goes through. Then we expect electron goes through slit 1 or 2, right? Every hour we get a new spot on the screen. • Interference pattern builds up slowly: ...
REVIEW OF WAVE MECHANICS
... profoundly disturb the state of a system. If the initial wave function of a system is described as a linear superposition of the eigenfunctions before the measurement, after the measurement it has been “reduced” or “collapsed” to one eigenfunction (assuming that we have performed a perfect ‘noise-fr ...
... profoundly disturb the state of a system. If the initial wave function of a system is described as a linear superposition of the eigenfunctions before the measurement, after the measurement it has been “reduced” or “collapsed” to one eigenfunction (assuming that we have performed a perfect ‘noise-fr ...
+l - My CCSD
... This image shows a ring of 76 iron atoms on a copper (111) surface. Electrons on this surface form a two-dimensional electron gas and scatter from the iron atoms but are confined by boundary or "corral." The wave pattern in the interior is due to the density distribution of the trapped electrons. Th ...
... This image shows a ring of 76 iron atoms on a copper (111) surface. Electrons on this surface form a two-dimensional electron gas and scatter from the iron atoms but are confined by boundary or "corral." The wave pattern in the interior is due to the density distribution of the trapped electrons. Th ...
- Physics
... Paschen series (infrared) What does classical physics predict for the future of an electron orbiting a proton? What are the two postulates of Bohr's theory for the Hydrogen atom? hf = Eupper - Elower What is true about the angular momentum of the electron in an atom? What is the principal quantum nu ...
... Paschen series (infrared) What does classical physics predict for the future of an electron orbiting a proton? What are the two postulates of Bohr's theory for the Hydrogen atom? hf = Eupper - Elower What is true about the angular momentum of the electron in an atom? What is the principal quantum nu ...
Chapter 7 Quantum Theory of the Atom
... measures the strength of its electric and magnetic fields related to the intensity of the radiation (how dim or bright) Notes: frequency and wavelength are inversely related as the frequency (ν ν) increases the wavelength (λ λ ) decreases ...
... measures the strength of its electric and magnetic fields related to the intensity of the radiation (how dim or bright) Notes: frequency and wavelength are inversely related as the frequency (ν ν) increases the wavelength (λ λ ) decreases ...
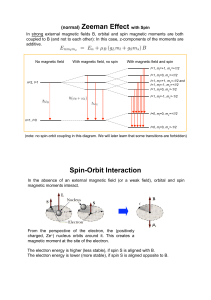
(normal) Zeeman Effect with Spin Spin
... (note: no spin-orbit coupling in this diagram. We will later learn that some transitions are forbidden) ...
... (note: no spin-orbit coupling in this diagram. We will later learn that some transitions are forbidden) ...