Material since exam 3
... a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
... a p-shell (except for He). How many electrons do next two inert gas atoms after helium ( neon (Ne) and argon (Ar) ) have. In this range of atomic number the subshells fill in order of increasing angular momentum. ...
6. Quantum Mechanics II
... Homework due next Wednesday Sept. 30th Read Chapters 4 and 5 of Kane Chapter 4: 2, 3, 5, 13, 20 problems Chapter 5: 3, 4, 5, 7, 8 problems ...
... Homework due next Wednesday Sept. 30th Read Chapters 4 and 5 of Kane Chapter 4: 2, 3, 5, 13, 20 problems Chapter 5: 3, 4, 5, 7, 8 problems ...
Presentation #2
... The approach of Hamilton is the one that best allows the transformation from classical to quantum mechanics. In conservative systems (in which the total energy remains constant with time) the Hamilton function is equal to the total energy of the system expressed in terms of the coordinates of the pa ...
... The approach of Hamilton is the one that best allows the transformation from classical to quantum mechanics. In conservative systems (in which the total energy remains constant with time) the Hamilton function is equal to the total energy of the system expressed in terms of the coordinates of the pa ...
Early Modern Physics
... • already went over kinematics • Rutherford scattering can either be off a heavier object (nuclei) change in angle but little energy loss “multiple scattering” • or off light target (electrons) where can transfer energy but little angular change (energy loss due to ionization, also produces “del ...
... • already went over kinematics • Rutherford scattering can either be off a heavier object (nuclei) change in angle but little energy loss “multiple scattering” • or off light target (electrons) where can transfer energy but little angular change (energy loss due to ionization, also produces “del ...
F = mv r
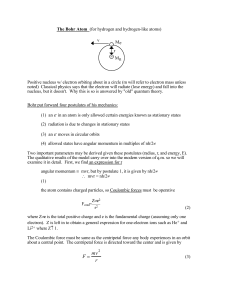
... Two important parameters may be derived given these postulates (radius, r, and energy, E). The qualitative results of the model carry over into the modern version of q.m. so we will examine it in detail. First, we find an expression for r angular momentum ≡ mvr, but by postulate 1, it is given by nh ...
... Two important parameters may be derived given these postulates (radius, r, and energy, E). The qualitative results of the model carry over into the modern version of q.m. so we will examine it in detail. First, we find an expression for r angular momentum ≡ mvr, but by postulate 1, it is given by nh ...
force on moving charge
... between two “particles”, a photon, and an electron. The way to do this is to use two important conservation laws: Conservation of energy, and of momentum. No energy or momentum is added to the system of two particles from the outside, so both should remain constant during the collision. Initially th ...
... between two “particles”, a photon, and an electron. The way to do this is to use two important conservation laws: Conservation of energy, and of momentum. No energy or momentum is added to the system of two particles from the outside, so both should remain constant during the collision. Initially th ...
pdf format
... Basis of exposure meters, night vision goggles etc... – This only happens when a high enough frequency (i.e. short enough wavelength) light is used, because a certain amount of energy is needed to free each electron from the metal and that energy must come from a single photon of high enough energy. ...
... Basis of exposure meters, night vision goggles etc... – This only happens when a high enough frequency (i.e. short enough wavelength) light is used, because a certain amount of energy is needed to free each electron from the metal and that energy must come from a single photon of high enough energy. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 27. a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on θ and φ. b) The wave function of 1s orbital of Li2+ is Ψ1s = (1/√π) (Z/a0)3/2 exp(-Zr/a0), where a0 is the m ...
... 27. a) Write the Schroedinger equation to be solved for H atom and solve it for its energy using a simple solution, which assumes the wave function to depend only on the distance r and not on θ and φ. b) The wave function of 1s orbital of Li2+ is Ψ1s = (1/√π) (Z/a0)3/2 exp(-Zr/a0), where a0 is the m ...
Waves in Motion
... charges Any object that is above absolute zero emits electromagnetic waves The entire range of possibilities is called the “Electromagnetic Spectrum” Still confused? Then click What are electromagnetic waves? To learn about the wavelength of photons click to the next slide. To move onto the EM spect ...
... charges Any object that is above absolute zero emits electromagnetic waves The entire range of possibilities is called the “Electromagnetic Spectrum” Still confused? Then click What are electromagnetic waves? To learn about the wavelength of photons click to the next slide. To move onto the EM spect ...
Stramski_IOCCG 2016_Interaction of Light and Matter
... • From Maxwell’s equations in differential form we obtain in free space ...
... • From Maxwell’s equations in differential form we obtain in free space ...
Spectra of Atoms
... How to explain these numerical series? Before we tackle that mystery, consider another: Mystery #5: Why don’t the electrons in an atom spiral into the nucleus? In classical electrodynamics, an accelerated charged particle ...
... How to explain these numerical series? Before we tackle that mystery, consider another: Mystery #5: Why don’t the electrons in an atom spiral into the nucleus? In classical electrodynamics, an accelerated charged particle ...
CLASSICAL FIELD THEORY AND ELECTRODYNAMICS
... (a) Derive the Euler-Lagrange equations of motion. Under what assumptions are they the Maxwell equations of electrodynamics? (b) Show explicitly, and with what assumptions, that Fermi’s Lagrangian density differs from ...
... (a) Derive the Euler-Lagrange equations of motion. Under what assumptions are they the Maxwell equations of electrodynamics? (b) Show explicitly, and with what assumptions, that Fermi’s Lagrangian density differs from ...
Quantum Mechanics
... 2. The hydrogen atom wave function may be written as R(r)Y`m (θ, φ), where R is the radial function and Y`m are the spherical harmonics. a. What is the differential equation for R(r)? b. The differential equation may be simplified somewhat by changing r and E into ρ = r/a0 and W = E/[ke2 /(2a0 )], w ...
... 2. The hydrogen atom wave function may be written as R(r)Y`m (θ, φ), where R is the radial function and Y`m are the spherical harmonics. a. What is the differential equation for R(r)? b. The differential equation may be simplified somewhat by changing r and E into ρ = r/a0 and W = E/[ke2 /(2a0 )], w ...
Chapter 5: Electrons In Atoms
... levels, and these electron lose energy by emitting light when they return to lower energy levels. Ordinary light is a mixture of all wavelengths, but light emitted by ...
... levels, and these electron lose energy by emitting light when they return to lower energy levels. Ordinary light is a mixture of all wavelengths, but light emitted by ...