Quanta to Quarks - The University of Sydney
... acceleration a. Estimate how much time it would take for an electron in an hydrogen atom to radiate away all its kinetic energy (assume electron moves on a constant orbit R ≈ 10-11 m). ...
... acceleration a. Estimate how much time it would take for an electron in an hydrogen atom to radiate away all its kinetic energy (assume electron moves on a constant orbit R ≈ 10-11 m). ...
Degeneracy of Hydrogen atom
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
... In quantum mechanics, an energy level is said to be degenerate if it corresponds to two or more different measurable states of a quantum system. Conversely, two or more different states of a quantum mechanical system are said to be degenerate if they give the same value of energy upon measurement. T ...
Foundations of Classical and Quantum Electrodynamics Brochure
... 4.2 The Motion of Charged Particles in Electromagnetic Fields. Transformation of the Electric Field 280 4.2.1 Interaction of Charged Particles with the Electromagnetic Field 280 4.2.2 Equations of Motion of a Relativistic Particle 282 4.2.3 Transformations of Electromagnetic Field Stress 288 4.2.4 D ...
... 4.2 The Motion of Charged Particles in Electromagnetic Fields. Transformation of the Electric Field 280 4.2.1 Interaction of Charged Particles with the Electromagnetic Field 280 4.2.2 Equations of Motion of a Relativistic Particle 282 4.2.3 Transformations of Electromagnetic Field Stress 288 4.2.4 D ...
Blackbody Radiation
... Solving the Schrodinger equation specifies Y (x,t) completely, except for a constant, ie. if Y is a solution, so is A xY . From the Born interpretation we have ||2.dx as the probability of finding the particle at position x.Since the particle must be somewhere the integral of this quantity from - ...
... Solving the Schrodinger equation specifies Y (x,t) completely, except for a constant, ie. if Y is a solution, so is A xY . From the Born interpretation we have ||2.dx as the probability of finding the particle at position x.Since the particle must be somewhere the integral of this quantity from - ...
Physical Chemistry II
... 4 φ is the work function of a metal, which is analogous to an ionization energy of an isolated atom. It typically has units of electron volts (eV) where 1 eV = 1.602 × 10−19 J 5 The Rydberg constant, R , is equal to 109677.581 cm−1 ...
... 4 φ is the work function of a metal, which is analogous to an ionization energy of an isolated atom. It typically has units of electron volts (eV) where 1 eV = 1.602 × 10−19 J 5 The Rydberg constant, R , is equal to 109677.581 cm−1 ...
Study Questions and Problems
... The values allowed for the three quantum numbers depend upon each other. The principal quantum n can have any integer value from 1, 2, 3, 4...to infinity. The secondary (or angular momentum) quantum number l can have values from 0, 1, 2, 3, to a maximum of n –1. The magnetic quantum number ml can ha ...
... The values allowed for the three quantum numbers depend upon each other. The principal quantum n can have any integer value from 1, 2, 3, 4...to infinity. The secondary (or angular momentum) quantum number l can have values from 0, 1, 2, 3, to a maximum of n –1. The magnetic quantum number ml can ha ...
Lecture 14 (Slides) September 27
... molecule does not change with time we can write simpler wave functions, such as Ψ(x,y,z) or Ψ(r,ϴ,φ). The classical analogy of a system with wave like behaviour not changing over time is any object that features standing waves. In such cases there is no destructive wave interference. Then wave like ...
... molecule does not change with time we can write simpler wave functions, such as Ψ(x,y,z) or Ψ(r,ϴ,φ). The classical analogy of a system with wave like behaviour not changing over time is any object that features standing waves. In such cases there is no destructive wave interference. Then wave like ...
QM L-6
... The same procedure can be used to obtain expectation value of any quantity : Potential energy V(x) which is a function of x. The expectation value for ‘p’ can not be calculated this way. According to uncertainty principle: Page 3 ...
... The same procedure can be used to obtain expectation value of any quantity : Potential energy V(x) which is a function of x. The expectation value for ‘p’ can not be calculated this way. According to uncertainty principle: Page 3 ...
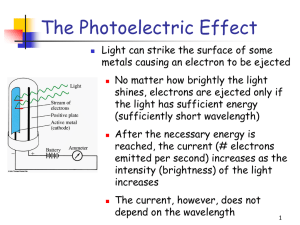
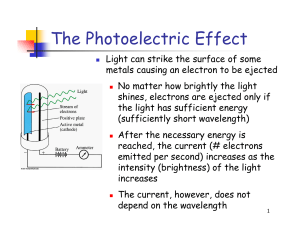
The Photoelectric Effect, work function
... Set a negative voltage on the anode. This will begin to repel the electrons. As the voltage increases, eventually even the fastest electrons are stopped/ turned back. This voltage is called the cutoff potential or stopping potential. The cutoff potential = the max. KE of the photoelectrons. Find Kma ...
... Set a negative voltage on the anode. This will begin to repel the electrons. As the voltage increases, eventually even the fastest electrons are stopped/ turned back. This voltage is called the cutoff potential or stopping potential. The cutoff potential = the max. KE of the photoelectrons. Find Kma ...
Particle in a box (PPT - 6.9MB)
... The Schrodinger equation is given above. 1. The wavefunction Ψ can be complex, so we should remember to take the Real part of Ψ. 2. Time-harmonic solutions to Schrodinger equation are of the form: 3. Ψ(x,t) is a measurable quantity and represents the probability distribution of finding the particle. ...
... The Schrodinger equation is given above. 1. The wavefunction Ψ can be complex, so we should remember to take the Real part of Ψ. 2. Time-harmonic solutions to Schrodinger equation are of the form: 3. Ψ(x,t) is a measurable quantity and represents the probability distribution of finding the particle. ...
Document
... E(x,t) = A cos(kx – wt – q) can be expressed by using complex numbers. Since exp(i) = cos() + i sin(), E(x,t) can be written: E(x,t) = Re { A exp[i(kx – wt – q)] } We often leave out 'Re'. ...
... E(x,t) = A cos(kx – wt – q) can be expressed by using complex numbers. Since exp(i) = cos() + i sin(), E(x,t) can be written: E(x,t) = Re { A exp[i(kx – wt – q)] } We often leave out 'Re'. ...
PowerPoint
... Atom has a number of discrete energy levels (orbits) in which an electron may revolve without emitting or absorbing electromagnetic radiation. As the orbital radius increases so does the energy of the electron ...
... Atom has a number of discrete energy levels (orbits) in which an electron may revolve without emitting or absorbing electromagnetic radiation. As the orbital radius increases so does the energy of the electron ...
Chapter 6
... Spin Quantum Number Symbolized by ms Indicates the fundamental spin states of an electron in an orbital Values are + ½ and -1/2 ...
... Spin Quantum Number Symbolized by ms Indicates the fundamental spin states of an electron in an orbital Values are + ½ and -1/2 ...
Physics 1020 Ch 10-12 Exam Answered
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...
... b. any electron present in an atom can have the same quantum state, since all electrons in an atom have the same mass and charge. c. there can be infinitely amount of electrons occupying an orbital as long as enough energy is provided. d. no two electrons can occupy the same quantum state. 11. The A ...