
N-Terminal Intramolecularly Conserved Histidines of Three Domains
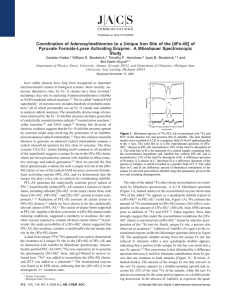
... alanine retained good activity at pH 6.3 (Table 3), while at all sites such individual substitutions resulted in greatly increased activities at pH 8 (Figure 4). Peptides with multiple substitutions also retained good specific activities at pH 6.3 (Table 3) but large increases of activity at pH 8, s ...
... alanine retained good activity at pH 6.3 (Table 3), while at all sites such individual substitutions resulted in greatly increased activities at pH 8 (Figure 4). Peptides with multiple substitutions also retained good specific activities at pH 6.3 (Table 3) but large increases of activity at pH 8, s ...
A SOLUBLE RIBONUCLEIC ACID INTERMEDIATE IN PROTEIN
... irreversibly into cY-peptide linkage in protein has been used in our laboratories for a number of years as a measure of protein synthesis. The essential components of this system are the microsomal ribonucleoprotein particles, certain enzymes derived from the soluble protein fraction, adenosine trip ...
... irreversibly into cY-peptide linkage in protein has been used in our laboratories for a number of years as a measure of protein synthesis. The essential components of this system are the microsomal ribonucleoprotein particles, certain enzymes derived from the soluble protein fraction, adenosine trip ...
The Cell, 5e
... sucrose as its primary incredients, the major carbohydrate products that enter the blood are which of the following: a. glucose b. fructose and galactose c. galactose and glucose d. fructose and glucose e. glucose, galactose and fructose ...
... sucrose as its primary incredients, the major carbohydrate products that enter the blood are which of the following: a. glucose b. fructose and galactose c. galactose and glucose d. fructose and glucose e. glucose, galactose and fructose ...
Cloning of genes from genomic DNA Parts 4 and 5: Ligation and
... overhangs) created from the restriction enzyme digestions will allow the XbaI end of the plasmid to basepair with the XbaI end of the PCR product. The HinDIII ends will also basepair to each other. Then DNA ligase will be able to form the phosphodiester bond between the two fragments, ligating them ...
... overhangs) created from the restriction enzyme digestions will allow the XbaI end of the plasmid to basepair with the XbaI end of the PCR product. The HinDIII ends will also basepair to each other. Then DNA ligase will be able to form the phosphodiester bond between the two fragments, ligating them ...
2. Biotechnology Booklet [A2]
... state. Dolly’s birth was a breakthrough, because it showed that the processes leading to cell specialisation are not irreversible; even specialised cells can be ‘reprogrammed’ into an embryonic state. While cloning seems relatively easy to achieve using this method, Dolly’s early death has raised co ...
... state. Dolly’s birth was a breakthrough, because it showed that the processes leading to cell specialisation are not irreversible; even specialised cells can be ‘reprogrammed’ into an embryonic state. While cloning seems relatively easy to achieve using this method, Dolly’s early death has raised co ...
Intended Use
... upon the use of malate dehydrogenase and NADH. Optimized procedures were presented by Henry3 in 1960 and Amador and Wacker4 in 1962. These modifications increased accuracy and lowered the effect of interfering substances. The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry an ...
... upon the use of malate dehydrogenase and NADH. Optimized procedures were presented by Henry3 in 1960 and Amador and Wacker4 in 1962. These modifications increased accuracy and lowered the effect of interfering substances. The Committee on Enzymes of the Scandinavian Society for Clinical Chemistry an ...
Nutritional biochemistry
... monosaccharides units, either same or different. They accounts for 35% of dietary carbohydrates. ...
... monosaccharides units, either same or different. They accounts for 35% of dietary carbohydrates. ...
Synthesis of a novel β-lactamase hydrolysis resistant penicillin analog
... -Lactams have been in clinical use for about 50 years. During that period the ability to produce lactamases has become widespread amongst pathogenic bacteria through the mechanism of plasmid exchange (7-9). As a result, the usefulness of the first generation of -lactam antibiotics has been consid ...
... -Lactams have been in clinical use for about 50 years. During that period the ability to produce lactamases has become widespread amongst pathogenic bacteria through the mechanism of plasmid exchange (7-9). As a result, the usefulness of the first generation of -lactam antibiotics has been consid ...
Print - Stroke
... reflects small amounts of either constitutive or metabolizable liquid. In conjunction with this we find a moderate lipase activity and an intense reaction for free fatty acids. Quite striking is the presence, in quantity, of reaction products for the enzymes necessary to catabolize free fatty acid t ...
... reflects small amounts of either constitutive or metabolizable liquid. In conjunction with this we find a moderate lipase activity and an intense reaction for free fatty acids. Quite striking is the presence, in quantity, of reaction products for the enzymes necessary to catabolize free fatty acid t ...
Preface 1 PDF
... matrix function is the balance between deposition and turnover of its many, varied protein components. Indeed, the spatially and temporally precise removal and remodeling of connective tissue is critical to several developmental, homeostatic, and reparative processes. However, if matrix turnover and ...
... matrix function is the balance between deposition and turnover of its many, varied protein components. Indeed, the spatially and temporally precise removal and remodeling of connective tissue is critical to several developmental, homeostatic, and reparative processes. However, if matrix turnover and ...
Cell respiration Practice
... 5) The prefix glyco- comes from a Greek word that means “sweet.” The suffix -lysis comes from a Greek word that means “to loosen.” How are the meanings of these word parts related to the meaning of glycolysis? 6) What does it mean to say that glycolysis is an anaerobic process? 7) What do all cells ...
... 5) The prefix glyco- comes from a Greek word that means “sweet.” The suffix -lysis comes from a Greek word that means “to loosen.” How are the meanings of these word parts related to the meaning of glycolysis? 6) What does it mean to say that glycolysis is an anaerobic process? 7) What do all cells ...
27. GE_7.27 Gluconeo.. - College of Pharmacy at Howard University
... Hexokinase, which catalyzes the entry of free glucose into the glycolytic pathway, is a regulatory enzyme. There are four isozymes (designated I to IV). Hexokinase II has a high affinity for glucose Muscle hexokinases I and II are allosteric ally inhibited by their product, glucose 6phosphat ...
... Hexokinase, which catalyzes the entry of free glucose into the glycolytic pathway, is a regulatory enzyme. There are four isozymes (designated I to IV). Hexokinase II has a high affinity for glucose Muscle hexokinases I and II are allosteric ally inhibited by their product, glucose 6phosphat ...
Lecture 27
... activated by N-acetylglutamate. N-acetylglutamate is synthesized from glutamate and acetylCoA by N-acetylglutamate synthase, it is hydrolyzed by a specific hydrolase. Rate of urea production is dependent on [N-acetylglutamate]. When aa breakdown rates increase, excess nitrogen must be excreted. This ...
... activated by N-acetylglutamate. N-acetylglutamate is synthesized from glutamate and acetylCoA by N-acetylglutamate synthase, it is hydrolyzed by a specific hydrolase. Rate of urea production is dependent on [N-acetylglutamate]. When aa breakdown rates increase, excess nitrogen must be excreted. This ...
1 Chapter 1 Chemistry On The Pyrimidine Ring
... The general mechanism for both of these enzymes first involves a sulfur transfer from a donor such as IscS for ThiI, and TusE for MnmA. This yields a protein persulfide intermediate. The enzymes then bind the tRNA and activates the uridine oxygen using ATP to form an adenylated intermediate. The su ...
... The general mechanism for both of these enzymes first involves a sulfur transfer from a donor such as IscS for ThiI, and TusE for MnmA. This yields a protein persulfide intermediate. The enzymes then bind the tRNA and activates the uridine oxygen using ATP to form an adenylated intermediate. The su ...
Sol: A process of physio
... It occurs in some organisms like some bacteria that produce lactic acid from pyruvic acid. In animal cells, such as muscles during exercise, when O2 is inadequate for cellular exercise, the pyruvic acid is reduced to lactic acid by lactate dehydrogenase. Reducing agent is NADH + H+ that is deoxidize ...
... It occurs in some organisms like some bacteria that produce lactic acid from pyruvic acid. In animal cells, such as muscles during exercise, when O2 is inadequate for cellular exercise, the pyruvic acid is reduced to lactic acid by lactate dehydrogenase. Reducing agent is NADH + H+ that is deoxidize ...
Glycogen Earth organisms use three major forms of - Rose
... reaction is driven physiologically by cleavage of pyrophosphate to inorganic phosphate by pyrophosphatase. The UDP-glucose pyrophosphorylase reaction acts as a priming step, and provides the energy required to form the glycoside bond in glycogen. Note that UTP is thus acting as a metabolic energy-co ...
... reaction is driven physiologically by cleavage of pyrophosphate to inorganic phosphate by pyrophosphatase. The UDP-glucose pyrophosphorylase reaction acts as a priming step, and provides the energy required to form the glycoside bond in glycogen. Note that UTP is thus acting as a metabolic energy-co ...
Cellular Respiration
... associated with hydrogen, especially in carbohydrates and fats • However, these fuels don’t spontaneously combine with O2 because they lack the activation E • Enzymes lower the barrier of activation E, allowing these fuels to be oxidized slowly • The “fall” of e-s during respiration is stepwise, via ...
... associated with hydrogen, especially in carbohydrates and fats • However, these fuels don’t spontaneously combine with O2 because they lack the activation E • Enzymes lower the barrier of activation E, allowing these fuels to be oxidized slowly • The “fall” of e-s during respiration is stepwise, via ...
Cellular Respiration
... associated with hydrogen, especially in carbohydrates and fats • However, these fuels don’t spontaneously combine with O2 because they lack the activation E • Enzymes lower the barrier of activation E, allowing these fuels to be oxidized slowly • The “fall” of e-s during respiration is stepwise, via ...
... associated with hydrogen, especially in carbohydrates and fats • However, these fuels don’t spontaneously combine with O2 because they lack the activation E • Enzymes lower the barrier of activation E, allowing these fuels to be oxidized slowly • The “fall” of e-s during respiration is stepwise, via ...
Glycogen Storage Disease
... responsible for creating glycogen from glucose, transporting the glycogen to and from storage areas within cells, and extracting glucose from the glycogen as needed. • Both creating and tearing down the glycogen macromolecule are multistep processes requiring a different enzyme at each step. • If on ...
... responsible for creating glycogen from glucose, transporting the glycogen to and from storage areas within cells, and extracting glucose from the glycogen as needed. • Both creating and tearing down the glycogen macromolecule are multistep processes requiring a different enzyme at each step. • If on ...
Ketamalt® 50
... – sugars, starches, amino acids and propylene glycol. In addition, Ketamalt® contains DIASTASE, a natural starch converting enzyme that converts starches present in the rumen to simple sugars. Propylene glycol is only one source of oral glucose precursor used to reverse ketosis in ruminants. Althoug ...
... – sugars, starches, amino acids and propylene glycol. In addition, Ketamalt® contains DIASTASE, a natural starch converting enzyme that converts starches present in the rumen to simple sugars. Propylene glycol is only one source of oral glucose precursor used to reverse ketosis in ruminants. Althoug ...
The Endoplasmic Reticulum Glucosyltransferase
... status of glycoproteins, causing the release of the latter from the lectin anchors. The ensuing shuttle between Glc-containing, lectin-bound, and Glc- and lectin-free species, catalyzed by the opposing activities of GT and glucosidases I and II ends on acquisition of native compact conformations. Th ...
... status of glycoproteins, causing the release of the latter from the lectin anchors. The ensuing shuttle between Glc-containing, lectin-bound, and Glc- and lectin-free species, catalyzed by the opposing activities of GT and glucosidases I and II ends on acquisition of native compact conformations. Th ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.



![2. Biotechnology Booklet [A2]](http://s1.studyres.com/store/data/000881801_1-016d6ea2a9343e8acf42bae0fafd1de9-300x300.png)


















