Fermentation Fermentation is an ancient mode of metabolism, and it
... and acetyl phosphate. As a fermentation pathway, it is employed mainly by the heterolactic acid bacteria, which include some species of Lactobacillus and Leuconostoc. In this pathway, glucose-phosphate is oxidized to 6-phosphogluconic acid, which becomes oxidized and decarboxylated to form pentose p ...
... and acetyl phosphate. As a fermentation pathway, it is employed mainly by the heterolactic acid bacteria, which include some species of Lactobacillus and Leuconostoc. In this pathway, glucose-phosphate is oxidized to 6-phosphogluconic acid, which becomes oxidized and decarboxylated to form pentose p ...
Unit 16: Understand the Principles and Carry Out the
... For P7, learners must review the effect of environmental changes on enzyme reaction rates. This may be as part of an investigation or as a literature review, covering the effects of temperature, pH, substrate and enzyme concentration and including construction and analysis of graphs of data. Suitabl ...
... For P7, learners must review the effect of environmental changes on enzyme reaction rates. This may be as part of an investigation or as a literature review, covering the effects of temperature, pH, substrate and enzyme concentration and including construction and analysis of graphs of data. Suitabl ...
Perspectives
... manner, and they fell into the same two groups, one producing a td-CRM and a second that did not (Suskind et al. 1955; Yanofsky and Bonner 1955; Yanofsky 1956; Suskind and Yanofsky 1961). From these findings it was evident that it should be possible to identify mutants producing many different td-CR ...
... manner, and they fell into the same two groups, one producing a td-CRM and a second that did not (Suskind et al. 1955; Yanofsky and Bonner 1955; Yanofsky 1956; Suskind and Yanofsky 1961). From these findings it was evident that it should be possible to identify mutants producing many different td-CR ...
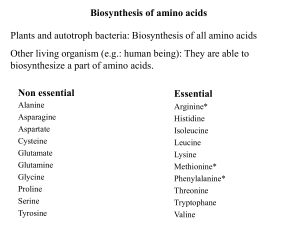
b. Ketogenic amino acids
... in the synthesis of glucose or lipid, or in the production of energy through their oxidation to CO2 and water by the citric acid cycle. ...
... in the synthesis of glucose or lipid, or in the production of energy through their oxidation to CO2 and water by the citric acid cycle. ...
Ancestral lipid biosynthesis and early membrane evolution
... constituted the earliest metabolic compartments before the emergence of cells [10,11]. In their model, a complex, geochemically driven, prebiotic chemistry developed, leading to different evolutionary stages (the ‘RNA’, ‘RNA–protein’ and ‘DNA’ eras) that included, successively, the invention of key ...
... constituted the earliest metabolic compartments before the emergence of cells [10,11]. In their model, a complex, geochemically driven, prebiotic chemistry developed, leading to different evolutionary stages (the ‘RNA’, ‘RNA–protein’ and ‘DNA’ eras) that included, successively, the invention of key ...
Lesson element
... least the poor quality and accuracy of some of the sources they have found as well as excellent examples of respiration. The tutor is a facilitator, guiding the learners in terms of research, identifying the important and relevant points and producing a lucid argument. In the final activity the tuto ...
... least the poor quality and accuracy of some of the sources they have found as well as excellent examples of respiration. The tutor is a facilitator, guiding the learners in terms of research, identifying the important and relevant points and producing a lucid argument. In the final activity the tuto ...
The ins and outs of sphingolipid synthesis
... sphingosine 1-phosphate phosphatase; SPL, sphingosine 1-phosphate lyase; DHCerS, dihydroceramide synthase; DHCD, dihydroceramide desaturase; CGalT, UDP-galactose:ceramide galactosyltransferase; CERT, ceramide transfer protein; SMS, SM synthase; GCS, GlcCer synthase; GCflip, GlcCer flippase; GalT1, L ...
... sphingosine 1-phosphate phosphatase; SPL, sphingosine 1-phosphate lyase; DHCerS, dihydroceramide synthase; DHCD, dihydroceramide desaturase; CGalT, UDP-galactose:ceramide galactosyltransferase; CERT, ceramide transfer protein; SMS, SM synthase; GCS, GlcCer synthase; GCflip, GlcCer flippase; GalT1, L ...
NAME: AKALABU, MAUREEN CHIDINMA COURSE: BCH 301 MAT
... nucleotides, is the most important factor, as the genetic "instructions" are contained in that sequence. For these enzymes, on the other hand, the three-dimensional arrangement of the strand is the most important factor, as the particular structure, not the sequence of nucleotides, allows the strand ...
... nucleotides, is the most important factor, as the genetic "instructions" are contained in that sequence. For these enzymes, on the other hand, the three-dimensional arrangement of the strand is the most important factor, as the particular structure, not the sequence of nucleotides, allows the strand ...
Plant cell walls to ethanol
... fucose. For example, the primary cell walls of conifers (i.e. softwoods) contain 10 % (w/w) xyloglucans and the primary cell walls of dicots (e.g. hardwoods) contain 20–25 % (w/w). Xylans are the most abundant class of hemicelluloses, with glucuronoarabinoxylan being the main target for enzymatic sa ...
... fucose. For example, the primary cell walls of conifers (i.e. softwoods) contain 10 % (w/w) xyloglucans and the primary cell walls of dicots (e.g. hardwoods) contain 20–25 % (w/w). Xylans are the most abundant class of hemicelluloses, with glucuronoarabinoxylan being the main target for enzymatic sa ...
NMR spectroscopy brings invisible protein states into
... when in states A and B, denoted by ωA stochastically between two conformational states, A and B, as a function of time, with states A and B and ωB, respectively. In the absence of denoted by red and blue colors, respectively. Each molecule will interconvert with its own trajectory, and a single exam ...
... when in states A and B, denoted by ωA stochastically between two conformational states, A and B, as a function of time, with states A and B and ωB, respectively. In the absence of denoted by red and blue colors, respectively. Each molecule will interconvert with its own trajectory, and a single exam ...
proximo-distal increase of enzymic activity in the dorsal
... in the nucleus and tractus gracilis of normal dogs. In the present material, such swellings were found in dogs, rats and cats; they were more frequent in dogs and rats than in cats. All swellings had an intense staining with the LDH reaction (Fig. 3B). In accordance with the general increase of enzy ...
... in the nucleus and tractus gracilis of normal dogs. In the present material, such swellings were found in dogs, rats and cats; they were more frequent in dogs and rats than in cats. All swellings had an intense staining with the LDH reaction (Fig. 3B). In accordance with the general increase of enzy ...
Isoniazid Drug Resistance: Computational
... electrophilic species, which then attack various MTB enzymes. As a prodrug, INH requires activation and after passively diffuses through the mycobacterial envelope, is activated by MnCl2 and the catalase– peroxidase KatG, possibly into an isonicotinoyl radical or anion, which then forms adduct in th ...
... electrophilic species, which then attack various MTB enzymes. As a prodrug, INH requires activation and after passively diffuses through the mycobacterial envelope, is activated by MnCl2 and the catalase– peroxidase KatG, possibly into an isonicotinoyl radical or anion, which then forms adduct in th ...
Document
... Characteristics of Enzymes • Some functional enzymes (holoenzymes) consist of two parts • Apoenzyme (protein portion) • Cofactor (metal ion) or coenzyme (organic molecule often a vitamin) ...
... Characteristics of Enzymes • Some functional enzymes (holoenzymes) consist of two parts • Apoenzyme (protein portion) • Cofactor (metal ion) or coenzyme (organic molecule often a vitamin) ...
Amino Acid Metabolism
... Proteins constantly undergo breakdown and synthesis Total protein turnover in a well-fed, adult human is estimated at about 300 g/day, of which approximately 100 g is myofibrillar protein, 30 g is digestive enzymes, 20 g is small intestinal cell protein, and 15 g is hemoglobin The remainder is accou ...
... Proteins constantly undergo breakdown and synthesis Total protein turnover in a well-fed, adult human is estimated at about 300 g/day, of which approximately 100 g is myofibrillar protein, 30 g is digestive enzymes, 20 g is small intestinal cell protein, and 15 g is hemoglobin The remainder is accou ...
How do changes in temperature, pH, enzyme concentration, and
... 2H2O2 (aq) > 2H2O(l) + O2 (g) (FAST) In this investigation, you will conduct experiments to determine how different environmental conditions affect the speed (rate) at which catalase converts hydrogen peroxide into water and oxygen gas. In this investigation, you will use catalase from liver. Remem ...
... 2H2O2 (aq) > 2H2O(l) + O2 (g) (FAST) In this investigation, you will conduct experiments to determine how different environmental conditions affect the speed (rate) at which catalase converts hydrogen peroxide into water and oxygen gas. In this investigation, you will use catalase from liver. Remem ...
Malate Dehydrogenase
... R109 and R171) which are important for substrate binding and catalysis. R102 and R109 are on the underside of the mobile loop and interact with substrate in ternary complex. The quanidium side chains of R102 and R171 form counterions for the substrate carboxylate groups, which contribute to binding ...
... R109 and R171) which are important for substrate binding and catalysis. R102 and R109 are on the underside of the mobile loop and interact with substrate in ternary complex. The quanidium side chains of R102 and R171 form counterions for the substrate carboxylate groups, which contribute to binding ...
Mechanism of Thymidylate Synthase, Cont`d
... Dehydrogenase • GAPDH is one of the key enzymes for glycolysis, reversibly catalyzes the first glycolytic reaction to involve oxidation-reduction • It converts the glyceraldehyde-3-phosphate (G3P) into the high energy phosphate compound, 1,3 bisphosphoglycerate (BPG), using NAD+ as a cofactor • BPG ...
... Dehydrogenase • GAPDH is one of the key enzymes for glycolysis, reversibly catalyzes the first glycolytic reaction to involve oxidation-reduction • It converts the glyceraldehyde-3-phosphate (G3P) into the high energy phosphate compound, 1,3 bisphosphoglycerate (BPG), using NAD+ as a cofactor • BPG ...
anaerobic and aerobic respiration
... Catabolism (reactions in which energy is harvested as chemical compounds are broken down.) refers to the exergonic process by which energy released by the breakdown of organic compounds such as glucose can be used to synthesize adenosine triphosphate (ATP), the form of energy required to do cellular ...
... Catabolism (reactions in which energy is harvested as chemical compounds are broken down.) refers to the exergonic process by which energy released by the breakdown of organic compounds such as glucose can be used to synthesize adenosine triphosphate (ATP), the form of energy required to do cellular ...
Enzyme

Enzymes /ˈɛnzaɪmz/ are macromolecular biological catalysts. Enzymes accelerate, or catalyze, chemical reactions. The molecules at the beginning of the process are called substrates and the enzyme converts these into different molecules, called products. Almost all metabolic processes in the cell need enzymes in order to occur at rates fast enough to sustain life. The set of enzymes made in a cell determines which metabolic pathways occur in that cell. The study of enzymes is called enzymology.Enzymes are known to catalyze more than 5,000 biochemical reaction types. Most enzymes are proteins, although a few are catalytic RNA molecules. Enzymes' specificity comes from their unique three-dimensional structures.Like all catalysts, enzymes increase the rate of a reaction by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur many millions of times faster. An extreme example is orotidine 5'-phosphate decarboxylase, which allows a reaction that would otherwise take millions of years to occur in milliseconds. Chemically, enzymes are like any catalyst and are not consumed in chemical reactions, nor do they alter the equilibrium of a reaction. Enzymes differ from most other catalysts by being much more specific. Enzyme activity can be affected by other molecules: inhibitors are molecules that decrease enzyme activity, and activators are molecules that increase activity. Many drugs and poisons are enzyme inhibitors. An enzyme's activity decreases markedly outside its optimal temperature and pH.Some enzymes are used commercially, for example, in the synthesis of antibiotics. Some household products use enzymes to speed up chemical reactions: enzymes in biological washing powders break down protein, starch or fat stains on clothes, and enzymes in meat tenderizer break down proteins into smaller molecules, making the meat easier to chew.