Enzymes
... • The digestion products, sugars, fatty acids, glycerol, amino acids, and dipeptides are able to enter the intestinal wall. • The amino acids and sugars then pass from the villi wall into the bloodstream and go to the liver. • Most of the fatty acids and monoacylglycerol are then reesterified and in ...
... • The digestion products, sugars, fatty acids, glycerol, amino acids, and dipeptides are able to enter the intestinal wall. • The amino acids and sugars then pass from the villi wall into the bloodstream and go to the liver. • Most of the fatty acids and monoacylglycerol are then reesterified and in ...
Visualizing Macromolecules
... how many different amino acid combinations are possible? ____________________ . For a tripeptide, how many different combinations are possible? ________________. Determine the number of different combinations of a tetrapeptide? ____________________________ . Clearly the number of possible combinatio ...
... how many different amino acid combinations are possible? ____________________ . For a tripeptide, how many different combinations are possible? ________________. Determine the number of different combinations of a tetrapeptide? ____________________________ . Clearly the number of possible combinatio ...
Metabolic Acidosis
... Utilization of Lactic Acid Lactate itself cannot be utilized by the body, and blood Lactate levels are therefore dependent on pyruvate metabolism ...
... Utilization of Lactic Acid Lactate itself cannot be utilized by the body, and blood Lactate levels are therefore dependent on pyruvate metabolism ...
Ketone Body Metabolism
... zAfter the diet has been changed to lower blood glucose for 3 days, the brain gets 30% of its energy from ketone bodies. zAfter 40 days, this goes up to 70% (during the initial stages the brain does not burn ketones, since they are an important substrate for lipid synthesis in the brain). zThe brain ...
... zAfter the diet has been changed to lower blood glucose for 3 days, the brain gets 30% of its energy from ketone bodies. zAfter 40 days, this goes up to 70% (during the initial stages the brain does not burn ketones, since they are an important substrate for lipid synthesis in the brain). zThe brain ...
HH-Unit-1-PPQs - Dalkeith High School
... 11. A fragment of DNA was found to have 120 guanine bases and 60 adenine bases. What is the total number of sugar molecules in this fragment? A. 60 B. 90 C. 180 D. 360 12. How many adenine molecules are present in a DNA molecule of 2000 bases, it 20% of the base molecules are cytosine? A. 200 B. 300 ...
... 11. A fragment of DNA was found to have 120 guanine bases and 60 adenine bases. What is the total number of sugar molecules in this fragment? A. 60 B. 90 C. 180 D. 360 12. How many adenine molecules are present in a DNA molecule of 2000 bases, it 20% of the base molecules are cytosine? A. 200 B. 300 ...
7 | cellular respiration
... • Describe the overall result in terms of molecules produced in the breakdown of glucose by glycolysis • Compare the output of glycolysis in terms of ATP molecules and NADH molecules produced You have read that nearly all of the energy used by living cells comes to them in the bonds of the sugar, gl ...
... • Describe the overall result in terms of molecules produced in the breakdown of glucose by glycolysis • Compare the output of glycolysis in terms of ATP molecules and NADH molecules produced You have read that nearly all of the energy used by living cells comes to them in the bonds of the sugar, gl ...
Document
... They bind to substrates, but are never covalently attached to substrate or product. They increase the equilibrium constant for a reaction, thus favoring product formation. They increase the stability of the product of a desired reaction by allowing ionizations, resonance, and isomerizations not norm ...
... They bind to substrates, but are never covalently attached to substrate or product. They increase the equilibrium constant for a reaction, thus favoring product formation. They increase the stability of the product of a desired reaction by allowing ionizations, resonance, and isomerizations not norm ...
macromolecules
... Most monomers are joined by 1-4 linkages between the glucose molecules. Plants store starch within plastids, including chloroplasts. Animals, including human, have enzymes that can hydrolyze plant starch making glucose available for metabolism. ...
... Most monomers are joined by 1-4 linkages between the glucose molecules. Plants store starch within plastids, including chloroplasts. Animals, including human, have enzymes that can hydrolyze plant starch making glucose available for metabolism. ...
Cellular Energy and Enzymatic Function
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
Human Physiology - Maryville University
... pass thru ATP synthase to generate 1 ATP This yields 36-38 ATPs/glucose However some of these are used to pump ATPs out of mitochondria So net yield is 30-32 ATPs/glucose Really takes 4H+s to generate 1 exported ATP ...
... pass thru ATP synthase to generate 1 ATP This yields 36-38 ATPs/glucose However some of these are used to pump ATPs out of mitochondria So net yield is 30-32 ATPs/glucose Really takes 4H+s to generate 1 exported ATP ...
Three-Point Binding Model
... • First proposed by Ogsten (1948) to explain biological enantioselection/enantiospecificity • Serves as a model for chromatographic chiral stationary phases Preferential binding occurs via intramolecular non-covalent forces: H-bonding salt bridge Ionic Dipole-dipole Van der Waals ...
... • First proposed by Ogsten (1948) to explain biological enantioselection/enantiospecificity • Serves as a model for chromatographic chiral stationary phases Preferential binding occurs via intramolecular non-covalent forces: H-bonding salt bridge Ionic Dipole-dipole Van der Waals ...
WSFNR-17-13 Coder - Warnell School of Forestry and Natural
... fermentation occurs, like glycolysis, within the cytoplasm, and generates energy through electron transfer molecules. The energy generated under anaerobic conditions is about 4% normal amounts. At first under oxygen deficiencies, cells increase respiration rates (increase CO2 release) attempting to ...
... fermentation occurs, like glycolysis, within the cytoplasm, and generates energy through electron transfer molecules. The energy generated under anaerobic conditions is about 4% normal amounts. At first under oxygen deficiencies, cells increase respiration rates (increase CO2 release) attempting to ...
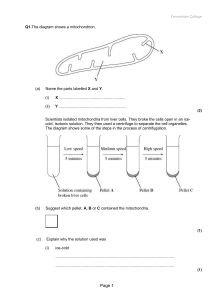
RespirationQuestions.doc - KS3, GCSE and A
... and C, on the electron transport chain in these mitochondria. In each of three experiments, a different inhibitor was added. The table shows the state of the electron carriers, W–Z, after the addition of inhibitor. ...
... and C, on the electron transport chain in these mitochondria. In each of three experiments, a different inhibitor was added. The table shows the state of the electron carriers, W–Z, after the addition of inhibitor. ...
II. Acids and Bases
... oxyanion (polyatomic ion with oxygen in it) a. Name the polyatomic ion. b. Replace “ate” with “ic”, “ite” with “ous.” c. Change nonmetal root for pronunciation. d. Add “acid” to the name. (e.g., H2SO4 = sulfuric acid) ...
... oxyanion (polyatomic ion with oxygen in it) a. Name the polyatomic ion. b. Replace “ate” with “ic”, “ite” with “ous.” c. Change nonmetal root for pronunciation. d. Add “acid” to the name. (e.g., H2SO4 = sulfuric acid) ...
lect21
... -in most cases the rate of the first reaction is 10 – 100 times the rate of the second reactions, but in some enzymes the rates are nearly equal ...
... -in most cases the rate of the first reaction is 10 – 100 times the rate of the second reactions, but in some enzymes the rates are nearly equal ...
11.lec11_biochemical-cycles - Lightweight OCW University of
... bacteria employ an enzyme, known as nitrogenase, to do the energy-intensive work of splitting the atmospheric nitrogen molecule into individual atoms for combination into other compounds. Nitrogen is also fixed by man-made processes, primarily industrial process that create ammonia and nitrogen-rich ...
... bacteria employ an enzyme, known as nitrogenase, to do the energy-intensive work of splitting the atmospheric nitrogen molecule into individual atoms for combination into other compounds. Nitrogen is also fixed by man-made processes, primarily industrial process that create ammonia and nitrogen-rich ...
Stoichiometry
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
... from the reaction of 0.10 mole of Mg3N2? • How many moles of NH3 would be produced from the reaction of 500. g of Mg3N2? • How many molecules of water would be required to react with 3.64 g of Mg3N2? • What is the maximum number of grams of Mg(OH)2 that can be produced by the reaction of 10.0 g of M ...
Biology: Concepts and Connections, 6e (Campbell)
... C) endergonic reactions can be fueled by coupling them with the hydrolysis of high-energy phosphate bonds in ATP. D) the regeneration of ATP from ADP can be fueled by coupling it with endergonic reactions. E) ATP is a disposable form of chemical energy, used once and then discarded by the cell. Answ ...
... C) endergonic reactions can be fueled by coupling them with the hydrolysis of high-energy phosphate bonds in ATP. D) the regeneration of ATP from ADP can be fueled by coupling it with endergonic reactions. E) ATP is a disposable form of chemical energy, used once and then discarded by the cell. Answ ...
tRNA - ISE
... of a peptide bond between the carboxyl group at the end of a growing polypeptide chain and a free amino group on an incoming amino acid ...
... of a peptide bond between the carboxyl group at the end of a growing polypeptide chain and a free amino group on an incoming amino acid ...
RESPIRATION IN PLANTS
... These hydrogen carriers enter the next phase known as the respiratory chain or Electron-Transport-Chain (E.T.C.) for further release of energy. The Respiratory Chain or Electron Transport Chain (E.T.C.) or OxidaivePhosphorylation z ...
... These hydrogen carriers enter the next phase known as the respiratory chain or Electron-Transport-Chain (E.T.C.) for further release of energy. The Respiratory Chain or Electron Transport Chain (E.T.C.) or OxidaivePhosphorylation z ...
What is Cellular Respiration?
... In respiration, glucose is oxidized and thus releases energy. Oxygen is reduced to form water. The carbon atoms of the sugar molecule are released as carbon dioxide (CO2). The complete breakdown of glucose to carbon dioxide and water requires two major steps: 1) glycolysis and 2) aerobic respiration ...
... In respiration, glucose is oxidized and thus releases energy. Oxygen is reduced to form water. The carbon atoms of the sugar molecule are released as carbon dioxide (CO2). The complete breakdown of glucose to carbon dioxide and water requires two major steps: 1) glycolysis and 2) aerobic respiration ...
Practice Exam III answers
... 3). For the reaction catalyzed by adenylate kinase: ATP + AMP 2 ADP The overall G’ 0 even though the cellular [AMP], [ADP], and [ATP] are far away from their equilibrium values. What is an alternative explanation for why this reaction operates with a G’ 0? a). Adenylate kinase is altering t ...
... 3). For the reaction catalyzed by adenylate kinase: ATP + AMP 2 ADP The overall G’ 0 even though the cellular [AMP], [ADP], and [ATP] are far away from their equilibrium values. What is an alternative explanation for why this reaction operates with a G’ 0? a). Adenylate kinase is altering t ...
darkreactions
... Last reaction is specific to Calvin cycle Others are found in gluconeogenesis or pentose phosphate pathway or both In this direction these reactions require the NADPH and ATP derived from the light reactions of photosynthesis ...
... Last reaction is specific to Calvin cycle Others are found in gluconeogenesis or pentose phosphate pathway or both In this direction these reactions require the NADPH and ATP derived from the light reactions of photosynthesis ...
Citric acid cycle
The citric acid cycle – also known as the tricarboxylic acid (TCA) cycle or the Krebs cycle – is a series of chemical reactions used by all aerobic organisms to generate energy through the oxidation of acetate derived from carbohydrates, fats and proteins into carbon dioxide and chemical energy in the form of adenosine triphosphate (ATP). In addition, the cycle provides precursors of certain amino acids as well as the reducing agent NADH that is used in numerous other biochemical reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest established components of cellular metabolism and may have originated abiogenically.The name of this metabolic pathway is derived from citric acid (a type of tricarboxylic acid) that is consumed and then regenerated by this sequence of reactions to complete the cycle. In addition, the cycle consumes acetate (in the form of acetyl-CoA) and water, reduces NAD+ to NADH, and produces carbon dioxide as a waste byproduct. The NADH generated by the TCA cycle is fed into the oxidative phosphorylation (electron transport) pathway. The net result of these two closely linked pathways is the oxidation of nutrients to produce usable chemical energy in the form of ATP.In eukaryotic cells, the citric acid cycle occurs in the matrix of the mitochondrion. In prokaryotic cells, such as bacteria which lack mitochondria, the TCA reaction sequence is performed in the cytosol with the proton gradient for ATP production being across the cell's surface (plasma membrane) rather than the inner membrane of the mitochondrion.