Worksheet – Measuring Heat
... 1. A fast-food item contains 544 nutritional Calories. Convert this energy to calories and to joules. (544,000 cal ; 2.28 x 106 J) 2. An endothermic process absorbs 138 kJ. How many calories of heat are absorbed?(3.30 x 104 cal) 3. An exothermic reaction releases 325000 calories. Convert this energy ...
... 1. A fast-food item contains 544 nutritional Calories. Convert this energy to calories and to joules. (544,000 cal ; 2.28 x 106 J) 2. An endothermic process absorbs 138 kJ. How many calories of heat are absorbed?(3.30 x 104 cal) 3. An exothermic reaction releases 325000 calories. Convert this energy ...
Thermal Energy
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
... b. Specific heat is the amount of heat required to raise the temperature of 1 kg of a material by one degree (C or K). 1) C water = 4184 J / kg C 2) C sand = 664 J / kg C ...
callister7e_sm_ch10_..
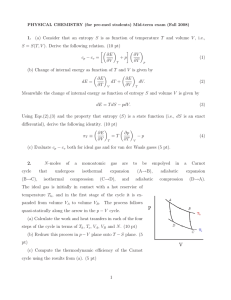
... 10.28 This problem asks that we briefly describe the simplest continuous cooling heat treatment procedure that would be used in converting a 4340 steel from one microstructure to another. Solutions to this problem require the use of Figure 10.28. (a) In order to convert from (martensite + ferrite + ...
... 10.28 This problem asks that we briefly describe the simplest continuous cooling heat treatment procedure that would be used in converting a 4340 steel from one microstructure to another. Solutions to this problem require the use of Figure 10.28. (a) In order to convert from (martensite + ferrite + ...
Sample Exam 3
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
... 9. The term “absolute zero” refers to a) the temperature at which water freezes. b) the temperature at which carbon dioxide freezes. c) the zero point in the Fahrenheit scale. d) the temperature at which all particle motion stops. e) the coldest temperature ever achieved on Earth. ...
10.213 Recitation April 8, 2002 A heat pump is used to heat a house
... April 8, 2002 A heat pump is used to heat a house in the winter and to cool it in the summer. During the winter, the outside air serves as a low-temperature heat source; during the summer, it acts as a hightemperature heat sink. The heat-transfer rate through the walls and roof of the house is 0.75 ...
... April 8, 2002 A heat pump is used to heat a house in the winter and to cool it in the summer. During the winter, the outside air serves as a low-temperature heat source; during the summer, it acts as a hightemperature heat sink. The heat-transfer rate through the walls and roof of the house is 0.75 ...
Chapter_3 - Weather Underground
... The air near the ground is warmed by ____________ What kind of conductor is air? Poor! ...
... The air near the ground is warmed by ____________ What kind of conductor is air? Poor! ...
The Impact on Design When Operating or Maintain Pipe
... method is used. As the specified maintain temperature approaches the T-Rating the allowable watt density for the tracer decreases resulting in a design with multiple tracers. This impacts the cost of the tracing system and in some instances results in a design that is impractical. On some occasions ...
... method is used. As the specified maintain temperature approaches the T-Rating the allowable watt density for the tracer decreases resulting in a design with multiple tracers. This impacts the cost of the tracing system and in some instances results in a design that is impractical. On some occasions ...
Lab 1: Temperature and Heat
... (h) Put your value for the latent heat of vaporization on the white board. When all lab groups have reported their values, calculate an average, standard deviation, and standard error. (i) Compare your result with the accepted value of 198 × 103 J/kg. How did your result compare with the accepted va ...
... (h) Put your value for the latent heat of vaporization on the white board. When all lab groups have reported their values, calculate an average, standard deviation, and standard error. (i) Compare your result with the accepted value of 198 × 103 J/kg. How did your result compare with the accepted va ...
Temperature Coefficient of Resistance
... that is the temperature coefficient of resistivity. If this behavior were actually a control designed by man, what would have driven him to dream up such an idea? The temperature of the conductor presents a problem in that once it reaches a critical point, the atomic bonds of the conductor will brea ...
... that is the temperature coefficient of resistivity. If this behavior were actually a control designed by man, what would have driven him to dream up such an idea? The temperature of the conductor presents a problem in that once it reaches a critical point, the atomic bonds of the conductor will brea ...
Physics 1301, Exam 4 Review
... Andersen 2006 Both problems are worth 15 points, and will be graded in a manner similar to the assigned homework problems in the book; up to 6 points possible for the description of your solution method, up to 6 points for your algebra and other work (available only if you receive the full 6 points ...
... Andersen 2006 Both problems are worth 15 points, and will be graded in a manner similar to the assigned homework problems in the book; up to 6 points possible for the description of your solution method, up to 6 points for your algebra and other work (available only if you receive the full 6 points ...























