Classification of Living Things PowerPoint File
... *also divided animals into 3 groups according to how they moved walking, flying, or swimming (land, air, or water) *his system was used from around 330 BC into the 1600's Name 2 animals for each of the three groups. What problems were there with his system? ...
... *also divided animals into 3 groups according to how they moved walking, flying, or swimming (land, air, or water) *his system was used from around 330 BC into the 1600's Name 2 animals for each of the three groups. What problems were there with his system? ...
Lecture 4: Heat transfer
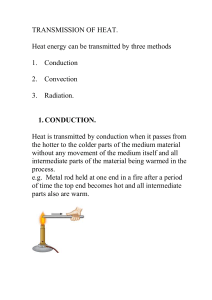
... Density is defined as mass per unit Volume. As the fluid is heated the Volume increases, the mass is constant therefore the density decreases. Hot fluids are less dense than cold fluids and will rise therefore convection currents (circular currents or movement within a fluid) due to different densit ...
... Density is defined as mass per unit Volume. As the fluid is heated the Volume increases, the mass is constant therefore the density decreases. Hot fluids are less dense than cold fluids and will rise therefore convection currents (circular currents or movement within a fluid) due to different densit ...
Thermal Energy - Issaquah Connect
... associated with the motion of objects (large or small objects). You can calculate the kinetic energy of an object of mass m with a velocity (speed) v from the formula K.E. = 1/2 mv^2. Thermal energy refers to the kinetic energy of the microscopic particles (atoms and molecules) that make up all samp ...
... associated with the motion of objects (large or small objects). You can calculate the kinetic energy of an object of mass m with a velocity (speed) v from the formula K.E. = 1/2 mv^2. Thermal energy refers to the kinetic energy of the microscopic particles (atoms and molecules) that make up all samp ...
Phase Changes and latent heat
... consider melting and boiling; both require energy input. There are attractive forces between molecules that must be overcome during melting g and boiling. g For a solid to melt,, the spring-like p g forces that hold molecules in place must be broken, and a certain amount of energy is required to bre ...
... consider melting and boiling; both require energy input. There are attractive forces between molecules that must be overcome during melting g and boiling. g For a solid to melt,, the spring-like p g forces that hold molecules in place must be broken, and a certain amount of energy is required to bre ...
Heat And Thermodynamics - Figure B
... volume also. Thus, heat is required for change in volume also. But if solid changes to liquid then change in volume is negligible and heat it required mainly to change the state only. Thus, latent heat of vaporization is more than latent heat of fusion. • After snow fall the temperature of atmospher ...
... volume also. Thus, heat is required for change in volume also. But if solid changes to liquid then change in volume is negligible and heat it required mainly to change the state only. Thus, latent heat of vaporization is more than latent heat of fusion. • After snow fall the temperature of atmospher ...
Part. A
... (b) Find an expression, as a function of temperature T, of the nuclear contribution to the molar internal entropy of the solid. Approximate the entropy in the low and the high T limits. (c) By directly counting the number of accessible states, calculate the nuclear entropy at the low and the high te ...
... (b) Find an expression, as a function of temperature T, of the nuclear contribution to the molar internal entropy of the solid. Approximate the entropy in the low and the high T limits. (c) By directly counting the number of accessible states, calculate the nuclear entropy at the low and the high te ...
Consider a rigid tank with a movable piston
... 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4) The exhaust process is r ...
... 1) The working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas. 2) All the processes that make up the cycle are internally reversible. 3) The combustion process is replaced by a heat-addition process from an external source. 4) The exhaust process is r ...
Relation between local temperature gradients and the direction of
... research. There are basically two motivations for this. On the one hand, the technological trend towards miniaturization of electronic circuits pushes for a better understanding of the mechanisms for heat production and energy flow at the microscopic level. On the other hand, from a more general poin ...
... research. There are basically two motivations for this. On the one hand, the technological trend towards miniaturization of electronic circuits pushes for a better understanding of the mechanisms for heat production and energy flow at the microscopic level. On the other hand, from a more general poin ...
CRYOGENICS
... The following methods are involved to produce the low temperature in cryogenics: Heat conduction: It is a relatively simple concept to understand. When two bodies are in contact, heat flows from the body with the higher temperature to the body with a lower temperature. Conduction can occur between a ...
... The following methods are involved to produce the low temperature in cryogenics: Heat conduction: It is a relatively simple concept to understand. When two bodies are in contact, heat flows from the body with the higher temperature to the body with a lower temperature. Conduction can occur between a ...
Homework #1 Solutions
... ∆U = Q + W The change in internal energy of a system comes about because of heat added to the system Q, plus the work done on the system. Heat and work represent energy in transit and are not thermodynamic state variables. Energy is a thermodynamic state variable. In thermodynamics the only two ways ...
... ∆U = Q + W The change in internal energy of a system comes about because of heat added to the system Q, plus the work done on the system. Heat and work represent energy in transit and are not thermodynamic state variables. Energy is a thermodynamic state variable. In thermodynamics the only two ways ...