Thermodynamics
... Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negative when it is done on the system. ...
... Q is positive when a system gains heat and negative when a system loses heat. Internal energy of a system can decrease if the system does work on its surroundings. Work is positive when it is done by the system and negative when it is done on the system. ...
Exercises - Madison County Schools
... 32. In order to quantify heat, we must specify the and of substance affected. 33. Suppose you place a pot with 1 cup of water and an identical pot with 2 cups of water on a hot stove for the same amount of time. Circle the letters beside the sentences that correctly describe what happens. a. More he ...
... 32. In order to quantify heat, we must specify the and of substance affected. 33. Suppose you place a pot with 1 cup of water and an identical pot with 2 cups of water on a hot stove for the same amount of time. Circle the letters beside the sentences that correctly describe what happens. a. More he ...
Chapter_03_Thermal_comfort_and_Heat_stess.pdf
... such cases since the body cannot absorb heat due to evaporation. (Perspiration can only cool the body, not heat the body.) In such cases, Eq. (3-45) cannot be used. The ratio of required to maximum evaporative cooling reflects whether the body is capable of maintaining equilibrium by evaporation, an ...
... such cases since the body cannot absorb heat due to evaporation. (Perspiration can only cool the body, not heat the body.) In such cases, Eq. (3-45) cannot be used. The ratio of required to maximum evaporative cooling reflects whether the body is capable of maintaining equilibrium by evaporation, an ...
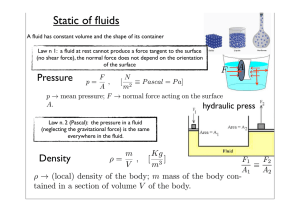
Static of fluids
... or give a quantity Q of heat where m is the mass and cL is the latent heat. ...
... or give a quantity Q of heat where m is the mass and cL is the latent heat. ...
Chemistry 1011
... • At any given temperature, only a fraction of molecules possess enough energy to react • Raising the temperature raises the average energy of the molecules. This substantially increases the number of molecules possessing the energy necessary to react ...
... • At any given temperature, only a fraction of molecules possess enough energy to react • Raising the temperature raises the average energy of the molecules. This substantially increases the number of molecules possessing the energy necessary to react ...
4.5 THERMAL ENERGY AND HEAT . PRACTICE
... 7. An electric room heater is best placed near the floor. In this way, the warm air rising from the heater by convection has an opportunity to be distributed throughout the room. If placed near the ceiling, the warm air would simply stay near the ceiling. 8. In most eases, the density of a substance ...
... 7. An electric room heater is best placed near the floor. In this way, the warm air rising from the heater by convection has an opportunity to be distributed throughout the room. If placed near the ceiling, the warm air would simply stay near the ceiling. 8. In most eases, the density of a substance ...
Thermodynamics–Honors
... Ability to do work Units– Joules (J), we will use “kJ” Can be converted to different types ...
... Ability to do work Units– Joules (J), we will use “kJ” Can be converted to different types ...