Name Date Class Adding Integers with the Same Sign Practice and
... Solve. The first one is done for you. 12. The temperature dropped 12F in 8 hours. If the final temperature was 7°F, what was the starting temperature? 5F ________________________________________________________________________________________ 13. At 3 P.M., the temperature was 9F. By 11 P.M., it ...
... Solve. The first one is done for you. 12. The temperature dropped 12F in 8 hours. If the final temperature was 7°F, what was the starting temperature? 5F ________________________________________________________________________________________ 13. At 3 P.M., the temperature was 9F. By 11 P.M., it ...
Joule`s Law and Heat Transfer Name:
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
... 7. Open DataStudio, select "Open Activity", select "Library", select "Physics Labs folder", and select "P16-Temperature and Heat". Click on the digits display and click start. 8. Plug in the power, and stir the water gently with the temperature sensor. 9. When the temperature reaches 20oC, the PC wi ...
Thermochemistry
... ◦ Energy is not created or destroyed in a physical or chemical process ◦ If energy in a system decreases, then the energy of the surroundings increases by the same amount ...
... ◦ Energy is not created or destroyed in a physical or chemical process ◦ If energy in a system decreases, then the energy of the surroundings increases by the same amount ...
Calorimetry and Specific Heat
... • Cup within a cup, air insulated • Obtain hot water in known amount with known temperature. • Put into known amount of antifreeze in cup of known mass and specific heat and stir • Heat lost by water = heat gained by AF and cup • mWcW(TW-TF) = mAcA(TF-TA) + mCcC(TF-TA) • cA = {mWcW(TW-TF) - mCcC(TF- ...
... • Cup within a cup, air insulated • Obtain hot water in known amount with known temperature. • Put into known amount of antifreeze in cup of known mass and specific heat and stir • Heat lost by water = heat gained by AF and cup • mWcW(TW-TF) = mAcA(TF-TA) + mCcC(TF-TA) • cA = {mWcW(TW-TF) - mCcC(TF- ...
PS5, Thermo Thermodynamics Standards: 3. Energy cannot be
... 9. Circle the letter of the source of the energy used by the heat engine to increase its internal energy. a. work output b. the sun c. low-temperature reservoir d. high-temperature reservoir 10. Is the following sentence true or false? Usable energy tends to become disorganized and unusable. 11. Cir ...
... 9. Circle the letter of the source of the energy used by the heat engine to increase its internal energy. a. work output b. the sun c. low-temperature reservoir d. high-temperature reservoir 10. Is the following sentence true or false? Usable energy tends to become disorganized and unusable. 11. Cir ...
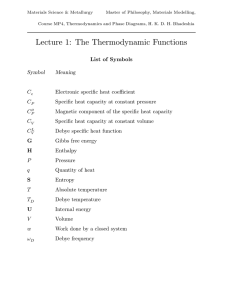
Thermodynamic functions - Phase Transformations Group
... The heat capacity can be determined experimentally using calorimetry. The data can then be related directly to the functions of state H, G and S. The heat capacity varies with temperature and other factors and hence is important in determining the stabilities of phase. It is useful to factorise the ...
... The heat capacity can be determined experimentally using calorimetry. The data can then be related directly to the functions of state H, G and S. The heat capacity varies with temperature and other factors and hence is important in determining the stabilities of phase. It is useful to factorise the ...
Test Thermodynamics Solutions
... 0th Law of thermodynamics: There exists a useful scalar quantity called temperature that is a property of all thermodynamics systems. When 2 bodies (A and B) are in thermal equilibrium (same temperature) with a third body (C), then all bodies (AC, BC, AB) are in equilibrium with each other. 1st Law ...
... 0th Law of thermodynamics: There exists a useful scalar quantity called temperature that is a property of all thermodynamics systems. When 2 bodies (A and B) are in thermal equilibrium (same temperature) with a third body (C), then all bodies (AC, BC, AB) are in equilibrium with each other. 1st Law ...
Heat Transfer Oil
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
... In many industrial applications heating is provided indirectly by circulating hot oil through a heat exchanger, thus reducing hot spots and increasing the safety of the heating process. In quenching applications, heat is required to be rapidly drawn away from the parts in contact with the oil. Due t ...
Buffet_geoneutrino - University of Hawaii Physics and Astronomy
... 1. Current temperature estimates yield high heat flow (Q > 6 TW) 2. Geodynamo may operate with lower heat flow i) = 0.1 TW implies Q ~ 2 TW ii) = 1.0 TW implies Q ~ 4.6 TW 3. Power requirements > 0.5 TW requires additional heat sources (200 ppm K is sufficient) -> gradual addition of heat sour ...
... 1. Current temperature estimates yield high heat flow (Q > 6 TW) 2. Geodynamo may operate with lower heat flow i) = 0.1 TW implies Q ~ 2 TW ii) = 1.0 TW implies Q ~ 4.6 TW 3. Power requirements > 0.5 TW requires additional heat sources (200 ppm K is sufficient) -> gradual addition of heat sour ...
Joule-Thomson Expansion
... So what can we conclude? Several things. First, since µ JT is positive at low temperatures and negative at high temperatures, it must have an inversion temperature. Second, the effect seems to depend (as we expected) on the attractive and repulsive forces acting between molecules. At low temperature ...
... So what can we conclude? Several things. First, since µ JT is positive at low temperatures and negative at high temperatures, it must have an inversion temperature. Second, the effect seems to depend (as we expected) on the attractive and repulsive forces acting between molecules. At low temperature ...