Problem set #1
... 2.1 The overhead vapor from a depropanizer distillation column is totally condensed in a water-cooled condenser at 120oF and 227 psig. The vapor is 95 mol % propane and 5 mol % isobutene. The vapor design flow rate is 25,500 lb/h and average latent heat of vaporization is 125 Btu/lb. Cooling water ...
... 2.1 The overhead vapor from a depropanizer distillation column is totally condensed in a water-cooled condenser at 120oF and 227 psig. The vapor is 95 mol % propane and 5 mol % isobutene. The vapor design flow rate is 25,500 lb/h and average latent heat of vaporization is 125 Btu/lb. Cooling water ...
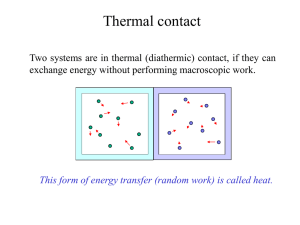
Temperature in Thermal Systems
... • Heat – Q, the energy that flows from one object to another because of a temperature difference. • Again from higher avg. KE to lower avg. KE. • Not another word for thermal energy. • Thermal energy in transit; measured also in Joules (J). • Three ways in which heat is transferred: • Conduction • C ...
... • Heat – Q, the energy that flows from one object to another because of a temperature difference. • Again from higher avg. KE to lower avg. KE. • Not another word for thermal energy. • Thermal energy in transit; measured also in Joules (J). • Three ways in which heat is transferred: • Conduction • C ...
The Functional Form of the Internal Energy
... potential energy. This energy comes at the expense of kinetic energy, therefore the molecules slow and the temperature decreases. Since both the temperature and pressure are decreasing as the gas expands, JT is positive. On the other hand, if repulsive forces dominate, as the gas expands potential ...
... potential energy. This energy comes at the expense of kinetic energy, therefore the molecules slow and the temperature decreases. Since both the temperature and pressure are decreasing as the gas expands, JT is positive. On the other hand, if repulsive forces dominate, as the gas expands potential ...
Model Question Paper – 1
... With suitable examples, distinguish between: i) Closed and open system ii) Path function and point function iii) Intensive and extensive properties A system of volume V contains a mass m of gas at a pressure p and temperature T. The macroscopic properties of the system obey the following relation: ( ...
... With suitable examples, distinguish between: i) Closed and open system ii) Path function and point function iii) Intensive and extensive properties A system of volume V contains a mass m of gas at a pressure p and temperature T. The macroscopic properties of the system obey the following relation: ( ...
MECHANICAL EQUIVALENT OF HEAT In this
... purely historical reasons, as the Mechanical Equivalent of Heat. These days we consider calories and joules simply as different units for energy. The apparatus consists of an aluminum cylinder that can be rotated by a crank, with a thermistor inside the cylinder to measure its temperature. A ...
... purely historical reasons, as the Mechanical Equivalent of Heat. These days we consider calories and joules simply as different units for energy. The apparatus consists of an aluminum cylinder that can be rotated by a crank, with a thermistor inside the cylinder to measure its temperature. A ...
Electrical Equivalent of Heat
... 5. Read both the voltmeter and the ammeter at intervals of one or two minutes keeping the water stirred so that the heat from the coil will not localize at any point. 6. Let the current flow until the temperature is the same number of degrees above the temperature of the room that it started below. ...
... 5. Read both the voltmeter and the ammeter at intervals of one or two minutes keeping the water stirred so that the heat from the coil will not localize at any point. 6. Let the current flow until the temperature is the same number of degrees above the temperature of the room that it started below. ...
Latent Heat
... of ice at -40°C, and we want to heat it to a temperature of 110°C. How much heat (energy) does that take? We know that when we heat the water from 0°C to 100°C, we can calculate how much heat is necessary to add in order to accomplish this by using Q = mcΔT. However, if we plot the heat added to the ...
... of ice at -40°C, and we want to heat it to a temperature of 110°C. How much heat (energy) does that take? We know that when we heat the water from 0°C to 100°C, we can calculate how much heat is necessary to add in order to accomplish this by using Q = mcΔT. However, if we plot the heat added to the ...
Project Meeting Minutes Template
... Series of curves for each part of model (curing, softening, hardening), all values of (T,h) above the curves meet conditions and are considered valid Time is implicit Choose the limiting point on plot at the smallest time, find cost relationship to time ...
... Series of curves for each part of model (curing, softening, hardening), all values of (T,h) above the curves meet conditions and are considered valid Time is implicit Choose the limiting point on plot at the smallest time, find cost relationship to time ...
List 8 Vocabulary Cards - Endeavor Charter School
... Transfer of heat through a liquid or a gas by rising and falling particles ...
... Transfer of heat through a liquid or a gas by rising and falling particles ...
the Animal kingdom
... Aquatic vertebrates with fins, scales, and gills Jawless fish Cartilaginous fish Bony fish ...
... Aquatic vertebrates with fins, scales, and gills Jawless fish Cartilaginous fish Bony fish ...
Thermodynamics - StrikerPhysics
... twice their original volume using two different processes. They are expanded isothermally and then, starting in the same initial state, they are expanded isobarically. During which process does the gas do more work? Find the work done in each case. ...
... twice their original volume using two different processes. They are expanded isothermally and then, starting in the same initial state, they are expanded isobarically. During which process does the gas do more work? Find the work done in each case. ...
Evolutionary Trends in Animals
... 6. The cells of all animals except sponges are organized into structural and functional units called ______________________. 7. In all animals except sponges, the zygote undergoes cell divisions forming a hollow ball of cells called a(n) ____________________. 8. The ______________________ is the lay ...
... 6. The cells of all animals except sponges are organized into structural and functional units called ______________________. 7. In all animals except sponges, the zygote undergoes cell divisions forming a hollow ball of cells called a(n) ____________________. 8. The ______________________ is the lay ...
Section3a - Lyle School of Engineering
... – After recrystallization is complete, the strain-free grains will continue to grow if the metal specimen is left at the elevated temperature - a phenomenon called grain growth. Grain growth does not need to be preceded by recovery and recrystallization; it may occur in all polycrystalline materials ...
... – After recrystallization is complete, the strain-free grains will continue to grow if the metal specimen is left at the elevated temperature - a phenomenon called grain growth. Grain growth does not need to be preceded by recovery and recrystallization; it may occur in all polycrystalline materials ...
Heat and Thermal Energy
... melts, and the water that comes from the ice will eventually have the same temperature as the bowl. This temperature will be lower than the original temperature of the bowl but higher than the original temperature of the ice cube. The water and the bowl end up at the same temperature because the par ...
... melts, and the water that comes from the ice will eventually have the same temperature as the bowl. This temperature will be lower than the original temperature of the bowl but higher than the original temperature of the ice cube. The water and the bowl end up at the same temperature because the par ...