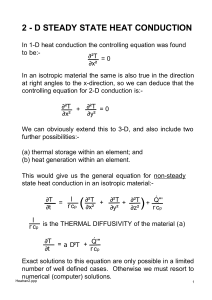
Fluids Thermo - Thermal Expansion
... a) Calculate how the flow of heat through a slab of material is affected by changes in the thickness or area of the slab, or the temperature difference between the two faces of the slab. b) Analyze what happens to the size and shape of an object when it is heated. ...
... a) Calculate how the flow of heat through a slab of material is affected by changes in the thickness or area of the slab, or the temperature difference between the two faces of the slab. b) Analyze what happens to the size and shape of an object when it is heated. ...
Mechanical Equivalent of Heat
... surroundings). This change from mechanical energy to thermal energy is equivalent to the thermal energy gained from heat. The relationship between temperature and thermal energy is given by the calorimeter equation: [2] ΔEth = mcΔT ΔEth = change in thermal energy m = mass of the substance in kg c = ...
... surroundings). This change from mechanical energy to thermal energy is equivalent to the thermal energy gained from heat. The relationship between temperature and thermal energy is given by the calorimeter equation: [2] ΔEth = mcΔT ΔEth = change in thermal energy m = mass of the substance in kg c = ...
Unit 3 Homework
... In the lectures, we obtained integral expressions for the specific heat of modes that can be approximated with a linear dispersion (Debye model) and constant dispersion (Einstein model). The ZA mode of graphene, which represents out-of-plane vibrations, is however closely approximated near the Brill ...
... In the lectures, we obtained integral expressions for the specific heat of modes that can be approximated with a linear dispersion (Debye model) and constant dispersion (Einstein model). The ZA mode of graphene, which represents out-of-plane vibrations, is however closely approximated near the Brill ...
thermodynamics
... temperature of the gold be after the system reaches equilibrium (cgold = 0.13 J/gAK)? (b) What is the apparent weight of the gold in the water? a. energy lost by gold = energy gained by water mc(Ti - Tf) = mc(Tf - Ti) 345 g A 0.13 J/gAK A (98.5 °C - Tf) = 656 g A 4.186 J/gAK A (Tf - 22.5 °C) 4417.72 ...
... temperature of the gold be after the system reaches equilibrium (cgold = 0.13 J/gAK)? (b) What is the apparent weight of the gold in the water? a. energy lost by gold = energy gained by water mc(Ti - Tf) = mc(Tf - Ti) 345 g A 0.13 J/gAK A (98.5 °C - Tf) = 656 g A 4.186 J/gAK A (Tf - 22.5 °C) 4417.72 ...
High Rate Lithium Cell
... Primary chemistry (non-rechargeable) High rate capability Advanced spiral-wound technology Stainless steel container Hermetic glass-to-metal sealing Wide operating temperature range as low as -55oC and up to +85oC Low self discharge rate (3% per year at 25oC) Restricted for transportation (Class 9) ...
... Primary chemistry (non-rechargeable) High rate capability Advanced spiral-wound technology Stainless steel container Hermetic glass-to-metal sealing Wide operating temperature range as low as -55oC and up to +85oC Low self discharge rate (3% per year at 25oC) Restricted for transportation (Class 9) ...
Thermoregulation contributes to homeostasis and involves anatomy
... The regulation of body temperature in humans is a complex system facilitated by feedback mechanisms. Nerve cells that control thermoregulation are concentrated in a brain region called the hypothalamus. The hypothalamus contains a group of nerve cells that functions as a thermostat. Nerve cells that ...
... The regulation of body temperature in humans is a complex system facilitated by feedback mechanisms. Nerve cells that control thermoregulation are concentrated in a brain region called the hypothalamus. The hypothalamus contains a group of nerve cells that functions as a thermostat. Nerve cells that ...
CHAPTER 10 NOTES FOR EIGHTH GRADE PHYSICAL SCIENCE
... CHAPTER 10 NOTES FOR EIGHTH GRADE PHYSICAL SCIENCE TEMPERATURE IS A MEASURE OF THE AVERAGE KINETIC ENERGY OF THE MOLECULES IN A SUBSTANCE. HEAT IS THE TRANSFER OF THERMAL ENERGY BETWEEN OBJECTS THAT ARE AT DIFFERENT TEMPERATURES. A THERMOMETER IS AN INSTRUMENT FOR MEASURING TEMERATURE. MERCURY AND A ...
... CHAPTER 10 NOTES FOR EIGHTH GRADE PHYSICAL SCIENCE TEMPERATURE IS A MEASURE OF THE AVERAGE KINETIC ENERGY OF THE MOLECULES IN A SUBSTANCE. HEAT IS THE TRANSFER OF THERMAL ENERGY BETWEEN OBJECTS THAT ARE AT DIFFERENT TEMPERATURES. A THERMOMETER IS AN INSTRUMENT FOR MEASURING TEMERATURE. MERCURY AND A ...
Statistical - Jordan University of Science and Technology
... positions. Let ε be the energy necessary to remove an atom from a lattice site to an interstitial position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c ...
... positions. Let ε be the energy necessary to remove an atom from a lattice site to an interstitial position and let n be the number of atoms occupying interstitial sites in equilibrium. a) what is the internal energy of the system. b) What is the total energy. Give an asymptotic formula when n>> 1? c ...
low temperature district heating system a modelling approach
... What is the problem In order to apply low-temperature concept for an existing district heating network: 1. Winter Time, Peak heat load periods 2. Summer Time, Low heat load periods There is need for new strategies in District Heating System. ...
... What is the problem In order to apply low-temperature concept for an existing district heating network: 1. Winter Time, Peak heat load periods 2. Summer Time, Low heat load periods There is need for new strategies in District Heating System. ...