Second review [Compatibility Mode]
... When 0.0300 mol of Na was added to 100.0 g of water, the temperature of the resulting solution rose from 25.0 oC to 37.9 oC. If the specific heat of the solution was 4.18 J g-1 K-1, calculate ? H, in kJ, for the reaction as written. ...
... When 0.0300 mol of Na was added to 100.0 g of water, the temperature of the resulting solution rose from 25.0 oC to 37.9 oC. If the specific heat of the solution was 4.18 J g-1 K-1, calculate ? H, in kJ, for the reaction as written. ...
Thermal Energy
... lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
... lower temperature • Heat does NOT transfer randomly • Heat only travels in ONE direction ...
Second-Order Linear Differential Equations
... differential equation, and P(x) ≠ 0, then the general solution is given by y(x) = c1y1(x) + c2y2(x), where c1 and c2 are arbitrary constants. The general solution to the differential equation is a linear combination of two linearly independent solutions. This means if we know two linearly independe ...
... differential equation, and P(x) ≠ 0, then the general solution is given by y(x) = c1y1(x) + c2y2(x), where c1 and c2 are arbitrary constants. The general solution to the differential equation is a linear combination of two linearly independent solutions. This means if we know two linearly independe ...
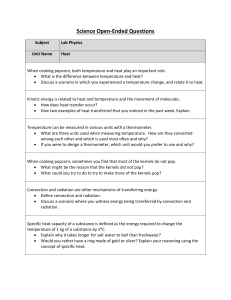
Heat and the Conservation of Energy
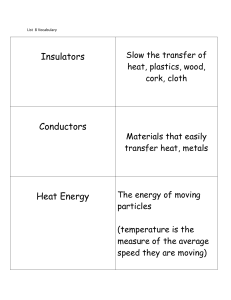
... Thermal conduction is when heat is passed along as the motion of one atom does work on an adjacent Conductors are materials that atom making it move conduct heat quickly Metals are good thermal conductors Ceramics, fiberglass etc do not, they are thermal insulators Liquids and Gases are good insulat ...
... Thermal conduction is when heat is passed along as the motion of one atom does work on an adjacent Conductors are materials that atom making it move conduct heat quickly Metals are good thermal conductors Ceramics, fiberglass etc do not, they are thermal insulators Liquids and Gases are good insulat ...
Thermocolour paper
... means of displaying temperature changes. The film responds quite rapidly. It can be used to illustrate many concepts linked with heat and temperature, including friction, conduction, insulation, feeling hot and cold, convection, radiation, evaporation and the greenhouse effect. Tips on its use Befor ...
... means of displaying temperature changes. The film responds quite rapidly. It can be used to illustrate many concepts linked with heat and temperature, including friction, conduction, insulation, feeling hot and cold, convection, radiation, evaporation and the greenhouse effect. Tips on its use Befor ...
PDF
... Equation (5) is a first order ordinary differential equation that when solved with the initial condition θ (0) = θ 0 , would give us the temperature of the spherical ball as a function of time. However, we made a large assumption in deriving Equation (5) - we assumed that the system is lumped. What ...
... Equation (5) is a first order ordinary differential equation that when solved with the initial condition θ (0) = θ 0 , would give us the temperature of the spherical ball as a function of time. However, we made a large assumption in deriving Equation (5) - we assumed that the system is lumped. What ...
DOC
... Equation (5) is a first order ordinary differential equation that when solved with the initial condition (0) 0 , would give us the temperature of the spherical ball as a function of time. However, we made a large assumption in deriving Equation (5) - we assumed that the system is lumped. What ...
... Equation (5) is a first order ordinary differential equation that when solved with the initial condition (0) 0 , would give us the temperature of the spherical ball as a function of time. However, we made a large assumption in deriving Equation (5) - we assumed that the system is lumped. What ...
9.1 Heat and Temperature
... III. Temperature A. A measurement expressing the average kinetic energy of the particles in a sample of matter (solid, liquid, or gas). 1. As Kinetic Energy of molecules increases, so does the temperature of that sample of matter. B. Temperature can be measured in Fahrenheit, Celsius, or in Kelvin. ...
... III. Temperature A. A measurement expressing the average kinetic energy of the particles in a sample of matter (solid, liquid, or gas). 1. As Kinetic Energy of molecules increases, so does the temperature of that sample of matter. B. Temperature can be measured in Fahrenheit, Celsius, or in Kelvin. ...
calculating specific heat capacity - Mikus
... When in contact with each other, objects at different temperatures transfer thermal energy until they reach the same temperature. This is called thermal equilibrium. Conservation of energy requires that the thermal energy lost by the hotter object as it cools be equal to the thermal energy gained by ...
... When in contact with each other, objects at different temperatures transfer thermal energy until they reach the same temperature. This is called thermal equilibrium. Conservation of energy requires that the thermal energy lost by the hotter object as it cools be equal to the thermal energy gained by ...
Conductionconvectionandradiation
... Heat energy can pass through solids, liquids and gases. Heat energy can be transferred from hot areas to cool areas in 3 ways. The 3 methods are ...
... Heat energy can pass through solids, liquids and gases. Heat energy can be transferred from hot areas to cool areas in 3 ways. The 3 methods are ...
16-2 - Laconia School District
... Thermometer an instrument for measuring temperature, often a sealed glass tube that contains a column of liquid, as mercury, that expands and contracts, or rises and falls, with temperature changes, the temperature being read where the top of the column coincides with a calibrated scale marked on t ...
... Thermometer an instrument for measuring temperature, often a sealed glass tube that contains a column of liquid, as mercury, that expands and contracts, or rises and falls, with temperature changes, the temperature being read where the top of the column coincides with a calibrated scale marked on t ...
Notes - hrsbstaff.ednet.ns.ca
... Example 2. Solving for specific heat capacity (c) Calculate the specific heat capacity of peanut oil if the mass is 65.0 grams, the initial temperature was 35.0oC and the final temperature was 5.2oC and the amount of heat was - 4000J (negative because it lost heat) Needing to find “c”, we must rearr ...
... Example 2. Solving for specific heat capacity (c) Calculate the specific heat capacity of peanut oil if the mass is 65.0 grams, the initial temperature was 35.0oC and the final temperature was 5.2oC and the amount of heat was - 4000J (negative because it lost heat) Needing to find “c”, we must rearr ...
Heat equation

The heat equation is a parabolic partial differential equation that describes the distribution of heat (or variation in temperature) in a given region over time.

![Second review [Compatibility Mode]](http://s1.studyres.com/store/data/003692853_1-a578e4717b0c8365c11d7e7f576654ae-300x300.png)