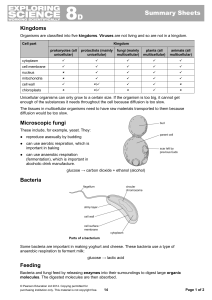
Eukaryotic heterotrophs
... where oxidized inorganic species e.g.. NO3- and SO42- act as electron acceptors in the absence of oxygen. – obligate anaerobes, facultative anaerobes ...
... where oxidized inorganic species e.g.. NO3- and SO42- act as electron acceptors in the absence of oxygen. – obligate anaerobes, facultative anaerobes ...
Glycolysis
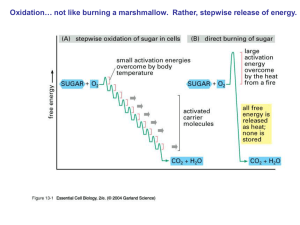
... • Trapped in chemical bonds of fats, proteins, and carbs (potential) • liberate energy – break bonds – release energy, CO2 and H20 – Energy is transferred to ATP for use in the body ...
... • Trapped in chemical bonds of fats, proteins, and carbs (potential) • liberate energy – break bonds – release energy, CO2 and H20 – Energy is transferred to ATP for use in the body ...
Oxidative phosphorylation (mitochondria)
... Two primary forms of energy are: Nucleotide triphosphate (e.g. ATP, GTP) Reducing power (NADH, NADPH) Two ways to make them: Through glycolysis (cytosol) Oxidative phosphorylation (mitochondria) ...
... Two primary forms of energy are: Nucleotide triphosphate (e.g. ATP, GTP) Reducing power (NADH, NADPH) Two ways to make them: Through glycolysis (cytosol) Oxidative phosphorylation (mitochondria) ...
Topic 2.5 Function
... • Consumers- do not contain photosynthetic pigments. Cannot make their own food • Decomposers- break down tissues of dead orgainc matter, release nutrients back into soil ...
... • Consumers- do not contain photosynthetic pigments. Cannot make their own food • Decomposers- break down tissues of dead orgainc matter, release nutrients back into soil ...
Cellular Respiration - Home - Mrs. Guida's AP Biology Class
... • Autotrophs vs Heterotrophs • Cellular Respiration- the oxidation of organic compounds to extract energy from chemical bonds ...
... • Autotrophs vs Heterotrophs • Cellular Respiration- the oxidation of organic compounds to extract energy from chemical bonds ...
BI 200 - Exam #2
... 23. In eukaryotic mitochondria enzymes of the Krebs cycle are found in the _________ and components of the electron transport chain are associated with the ____________. a. cristae; matrix b. cytoplasm; cristae c. matrix; cristae d. none of the above – eukaryotes don’t have mitochondria 24. In chem ...
... 23. In eukaryotic mitochondria enzymes of the Krebs cycle are found in the _________ and components of the electron transport chain are associated with the ____________. a. cristae; matrix b. cytoplasm; cristae c. matrix; cristae d. none of the above – eukaryotes don’t have mitochondria 24. In chem ...
Transport of molecules into a bacterial cell
... substrate (e.g. sugar) is used, making large amounts of product (e.g. lactic acid, ethanol, etc.) ...
... substrate (e.g. sugar) is used, making large amounts of product (e.g. lactic acid, ethanol, etc.) ...
Name__________________________ 1. Which of these
... is performed only by organisms that are incapable of photosynthesis. breaks down food molecules to release stored energy. occurs before plants are able to carry out photosynthesis. occurs only in animals. ...
... is performed only by organisms that are incapable of photosynthesis. breaks down food molecules to release stored energy. occurs before plants are able to carry out photosynthesis. occurs only in animals. ...
File
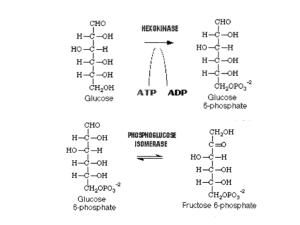
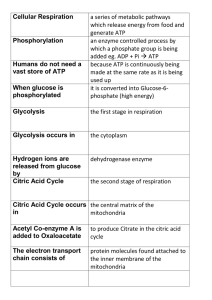
... which a phosphate group is being added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
... which a phosphate group is being added eg. ADP + Pi ATP because ATP is continuously being made at the same rate as it is being used up it is converted into Glucose-6phosphate (high energy) ...
Microbial Metabolism
... electrons are higher (more negative) on the tower • To determine which direction the reactions go, see which is “higher” on the electron tower • Note the position of important electron carriers (NAD, FAD, cytochrome a) and external electron donors/acceptors (H2, organic compounds, O2) ...
... electrons are higher (more negative) on the tower • To determine which direction the reactions go, see which is “higher” on the electron tower • Note the position of important electron carriers (NAD, FAD, cytochrome a) and external electron donors/acceptors (H2, organic compounds, O2) ...
B. Basic Concepts of Metabolism
... NAD(oxidized) + H+ + Pair of electrons NADH(reduced) FAD(oxidized) + H+ + Pair of electrons FADH(reduced) NAD and FAD are present only in small (catalytic) amounts – they cannot serve as the final electron acceptor, but must be regenerated (reoxidized) in order for metabolism to continue ...
... NAD(oxidized) + H+ + Pair of electrons NADH(reduced) FAD(oxidized) + H+ + Pair of electrons FADH(reduced) NAD and FAD are present only in small (catalytic) amounts – they cannot serve as the final electron acceptor, but must be regenerated (reoxidized) in order for metabolism to continue ...
Chemolithotrophs
... • Chemoorganotrophs: reduced inorganic electron donor for energy and electrons. • Chemolithotrophs: reduced inorganic electron donor for energy and electrons. • Phototrophs: use light energy and an electron donor molecule (H2O, H2S, organic). • Both may be autotrophs: fix CO2 into organic carbon via ...
... • Chemoorganotrophs: reduced inorganic electron donor for energy and electrons. • Chemolithotrophs: reduced inorganic electron donor for energy and electrons. • Phototrophs: use light energy and an electron donor molecule (H2O, H2S, organic). • Both may be autotrophs: fix CO2 into organic carbon via ...
Archaea
... molecule adenosine triphosphate (ATP). ATP is the principal ‘energy carrier’ of cells. Respiration may be either aerobic (in the presence of oxygen) or anaerobic (without oxygen). ...
... molecule adenosine triphosphate (ATP). ATP is the principal ‘energy carrier’ of cells. Respiration may be either aerobic (in the presence of oxygen) or anaerobic (without oxygen). ...
Anaerobic Respiration
... Some organisms, such as yeast and some bacteria, do not require oxygen and can survive on a less efficient way of getting energy Other organisms that generally require oxygen sometimes don’t have enough for all their cells to do aerobic respiration so they can use a less effiecent way of breaking do ...
... Some organisms, such as yeast and some bacteria, do not require oxygen and can survive on a less efficient way of getting energy Other organisms that generally require oxygen sometimes don’t have enough for all their cells to do aerobic respiration so they can use a less effiecent way of breaking do ...
Photosynthesis
... pyruvate, the end product of anaerobic glycolysis is lactate. Profit is 2 molecules of ATP. Enough energy for low-evolutional level organisms and probably for ...
... pyruvate, the end product of anaerobic glycolysis is lactate. Profit is 2 molecules of ATP. Enough energy for low-evolutional level organisms and probably for ...
Chemoheterotrophs Chemoheterotrophs: Fat β (beta)
... • Green & Purple Sulfur bacteria: Prototroph: a wild type strain of the same species, with all its genes intact, can grow without addition of that particular nutrient to the media ...
... • Green & Purple Sulfur bacteria: Prototroph: a wild type strain of the same species, with all its genes intact, can grow without addition of that particular nutrient to the media ...
Prokaryotes - AP Biology Overview
... o Chemoautotrophs – use inorganic chemicals in place of sunlight to manufacture organic compounds Heterotrophs o Photoheterotrophs – use light for energy, but must get carbon from an organic compound o Chemoheterotrophs – must consume to get energy and carbon ...
... o Chemoautotrophs – use inorganic chemicals in place of sunlight to manufacture organic compounds Heterotrophs o Photoheterotrophs – use light for energy, but must get carbon from an organic compound o Chemoheterotrophs – must consume to get energy and carbon ...
Metabolism
... • Redox potential is a measure of the affinity of compounds for electrons. The more positive a compound’s redox potential is, the greater its tendency to acquire electrons. – Redox potential is measured in millivolts (mV), relative to hydrogen at 1 atm pressure. Compounds are at 1 M concentration. • ...
... • Redox potential is a measure of the affinity of compounds for electrons. The more positive a compound’s redox potential is, the greater its tendency to acquire electrons. – Redox potential is measured in millivolts (mV), relative to hydrogen at 1 atm pressure. Compounds are at 1 M concentration. • ...
Bio260 Exam1.1 MW review
... • Define enzymes and their characteristics. – Understand the factors that influence enzyme activity: temperature, pH, substrate concentration, and inhibitors. – Understand competitive and noncompetitive inhibition. ...
... • Define enzymes and their characteristics. – Understand the factors that influence enzyme activity: temperature, pH, substrate concentration, and inhibitors. – Understand competitive and noncompetitive inhibition. ...
Groups of Organisms and their Interactions
... metabolism of organic compounds without the requirement for external electron acceptors energy derived from substrate-level phosphorylation low efficiency with incomplete metabolism of substrate e.g. glucose to ethanol ...
... metabolism of organic compounds without the requirement for external electron acceptors energy derived from substrate-level phosphorylation low efficiency with incomplete metabolism of substrate e.g. glucose to ethanol ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)