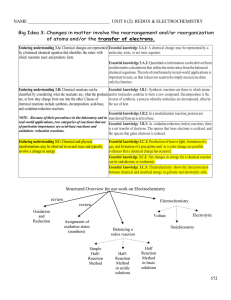
Gluconeogenesis by Dr Tarek
... PC glycolysis is inhibited and gluconeogenesis is activited • During starvation, the priority is to conserve blood glucose for the brain and muscle. Thus, under these conditions, PK in the liver is switched off. This occurs because the hormone glucagon is secreted into the bloodstream and activates ...
... PC glycolysis is inhibited and gluconeogenesis is activited • During starvation, the priority is to conserve blood glucose for the brain and muscle. Thus, under these conditions, PK in the liver is switched off. This occurs because the hormone glucagon is secreted into the bloodstream and activates ...
Chapter 24
... Glucose is converted to two molecules of pyruvate. An anaerobic reaction in cytoplasm. ...
... Glucose is converted to two molecules of pyruvate. An anaerobic reaction in cytoplasm. ...
The energy equivalents of ATP and the energy values of food
... 2. Heats of combustion of food proteins and fats derived from compositional data were within 1 % of published values obtained by calorimetry. 3. Cytoplasmic ATP equivalents for glucose, fat and protein range from 9.0 to 14.7,8.6 to 14.6 and 6.4 to 13.2mol cytoplasmic ATP/MJ of metabolizable energy r ...
... 2. Heats of combustion of food proteins and fats derived from compositional data were within 1 % of published values obtained by calorimetry. 3. Cytoplasmic ATP equivalents for glucose, fat and protein range from 9.0 to 14.7,8.6 to 14.6 and 6.4 to 13.2mol cytoplasmic ATP/MJ of metabolizable energy r ...
Answers - Pearson-Global
... example) of water with the coloured liquids introduced into the bottom of them. A simple observation of the progress of the colours up the tubes would be enough. There could be some problems if the liquids varied markedly in colour intensity. A student suggesting that you might put some white card o ...
... example) of water with the coloured liquids introduced into the bottom of them. A simple observation of the progress of the colours up the tubes would be enough. There could be some problems if the liquids varied markedly in colour intensity. A student suggesting that you might put some white card o ...
Proton transfer pathways and mechanism in bacterial reaction centers Minireview
... between the acid groups likely serve as bridges or connectors providing the missing links for hydrogen bonding chains. The pathways for proton transfer are functionally robust and relatively insensitive to perturbations. This is shown by the observation that mutation of a single Asp residue (AspM17 ...
... between the acid groups likely serve as bridges or connectors providing the missing links for hydrogen bonding chains. The pathways for proton transfer are functionally robust and relatively insensitive to perturbations. This is shown by the observation that mutation of a single Asp residue (AspM17 ...
ADP
... To produce NADPH (1) NADPH is the donor of hydrogen for various anabolic metabolism in organism (2) NADPH can participate in the hydroxylation reaction, involving biosynthesis or biotransformation in organism ...
... To produce NADPH (1) NADPH is the donor of hydrogen for various anabolic metabolism in organism (2) NADPH can participate in the hydroxylation reaction, involving biosynthesis or biotransformation in organism ...
respiration - A-level Biology Tutor
... Many failed to do this and so did not appreciate the important points required by the question. Although there were some good responses many candidates described only the breakdown of pyruvic acid into lactic acid. In failing to describe the pathway of conversion of glucose to pyruvate they were una ...
... Many failed to do this and so did not appreciate the important points required by the question. Although there were some good responses many candidates described only the breakdown of pyruvic acid into lactic acid. In failing to describe the pathway of conversion of glucose to pyruvate they were una ...
Nucleotides: Synthesis and Degradation
... Ingested nucleic acids are degraded to nucleotides by pancreatic nucleases, and intestinal phosphodiesterases in the intestine Group-specific nucleotidases and non-specific phosphatases degrade nucleotides into nucleosides – Direct absorption of nucleosides – Further degradation ...
... Ingested nucleic acids are degraded to nucleotides by pancreatic nucleases, and intestinal phosphodiesterases in the intestine Group-specific nucleotidases and non-specific phosphatases degrade nucleotides into nucleosides – Direct absorption of nucleosides – Further degradation ...
Acute nutritional ketosis: implications for exercise performance and metabolism Open Access
... muscular work in humans have been confounded by the inability to elevate ketone concentrations without the effects of starvation [64,65] or elevated fatty acids [66]. This lack of facility to induce acute ketosis has meant that all of the published literature methods to study fuel selection during k ...
... muscular work in humans have been confounded by the inability to elevate ketone concentrations without the effects of starvation [64,65] or elevated fatty acids [66]. This lack of facility to induce acute ketosis has meant that all of the published literature methods to study fuel selection during k ...
Biochemistry of Ensiling - DigitalCommons@University of Nebraska
... for feeding. Complications arise because: 1. There are always aerobic periods at the start and end of the ensiling process. 2. Simple sugars are not the only substrates metabolized. 3. Plant enzymes and other microbial species apart from LAB compete for substrate. The complexity of ensilage is incre ...
... for feeding. Complications arise because: 1. There are always aerobic periods at the start and end of the ensiling process. 2. Simple sugars are not the only substrates metabolized. 3. Plant enzymes and other microbial species apart from LAB compete for substrate. The complexity of ensilage is incre ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Document
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
... group is MORE positive than it was in the C-O-H group By increasing the number of highly electronegative O, more electrons were drawn away from that C, making it more positive. ...
Urea Cycle - MBBS Students Club
... Formation of citrulline • Ornithine and citrulline are basic amino acids that participate in the urea cycle. • (They are not incorporated into cellular proteins, because there are no codons for these amino acids) • Ornithine is regenerated with each turn of the urea cycle, much in the same way that ...
... Formation of citrulline • Ornithine and citrulline are basic amino acids that participate in the urea cycle. • (They are not incorporated into cellular proteins, because there are no codons for these amino acids) • Ornithine is regenerated with each turn of the urea cycle, much in the same way that ...
Intro to Metabolism II and Glycolysis
... conformational change occurs and glucose is released on the inside. This occurs via facilitated diffusion. c. Glucose follows a concentration gradient to enter a cell. As glucose concentration increases, you will see the transporters become saturated d. There are four glucose transporters that our b ...
... conformational change occurs and glucose is released on the inside. This occurs via facilitated diffusion. c. Glucose follows a concentration gradient to enter a cell. As glucose concentration increases, you will see the transporters become saturated d. There are four glucose transporters that our b ...
FREE Sample Here
... Answer: The three types of eukaryotic microbes are fungi, protozoa, and algae. Because they are all composed of eukaryotic cells, they have basic similarities in cellular structure, including the presence of a nucleus. However, these types of microbes differ in many ways as well. In terms of their n ...
... Answer: The three types of eukaryotic microbes are fungi, protozoa, and algae. Because they are all composed of eukaryotic cells, they have basic similarities in cellular structure, including the presence of a nucleus. However, these types of microbes differ in many ways as well. In terms of their n ...
Gluconeogenesis
... starvation is mainly amino acid catabolism. Some amino acids are catabolized to pyruvate, oxaloacetate, or precursors of these. Muscle proteins may break down to supply amino acids. These are transported to liver where they are deaminated and converted to gluconeogenesis inputs. Glycerol, derived fr ...
... starvation is mainly amino acid catabolism. Some amino acids are catabolized to pyruvate, oxaloacetate, or precursors of these. Muscle proteins may break down to supply amino acids. These are transported to liver where they are deaminated and converted to gluconeogenesis inputs. Glycerol, derived fr ...
Energy and cellular metabolism
... concentration side of a concentration gradient stores potential energy because it has the potential energy to move down the gradient. In chemical bonds, potential energy is stored in the position of the electrons that form the bond [p. 33]. [To learn more about kinetic and potential energy, see Ap ...
... concentration side of a concentration gradient stores potential energy because it has the potential energy to move down the gradient. In chemical bonds, potential energy is stored in the position of the electrons that form the bond [p. 33]. [To learn more about kinetic and potential energy, see Ap ...
Nucleotide Metabolism - Oregon State University
... Inosine is Converted to Hypoxanthine and Ribose-1P by a Purine Phosphorylase Hypoxanthine (Xanthine Oxidase) and Guanine (Guanase) are Converted to Xanthine Xanthine is Converted to Uric Acid by Xanthine Oxidase Uric Acid Crystals are the Cause of Gout Gout Treated With the Xanthine Oxidase Inhibito ...
... Inosine is Converted to Hypoxanthine and Ribose-1P by a Purine Phosphorylase Hypoxanthine (Xanthine Oxidase) and Guanine (Guanase) are Converted to Xanthine Xanthine is Converted to Uric Acid by Xanthine Oxidase Uric Acid Crystals are the Cause of Gout Gout Treated With the Xanthine Oxidase Inhibito ...
Karbohidrat Metabolizması
... by adenine nucleotides. Phosphofructokinase (Glycolysis) is inhibited by ATP and stimulated by AMP. Fructose-1,6-bisphosphatase (Gluconeogenesis) is inhibited by AMP. This insures that when cellular ATP is high (AMP would then be low), glucose is not degraded to make ATP. It is more useful to th ...
... by adenine nucleotides. Phosphofructokinase (Glycolysis) is inhibited by ATP and stimulated by AMP. Fructose-1,6-bisphosphatase (Gluconeogenesis) is inhibited by AMP. This insures that when cellular ATP is high (AMP would then be low), glucose is not degraded to make ATP. It is more useful to th ...
Mittenthal, J.E., Clarke, B., Waddell, T., and Fawcett, G.
... The g-reactions of a C-paranet redistribute the carbon atoms in the reacting metabolites. Each of these metabolites is only speci"ed by the number of carbon atoms it contains. Conversion of a Cto an R-paranet proceeds through the following stages, here as in our work on the pentose phosphate pathway ...
... The g-reactions of a C-paranet redistribute the carbon atoms in the reacting metabolites. Each of these metabolites is only speci"ed by the number of carbon atoms it contains. Conversion of a Cto an R-paranet proceeds through the following stages, here as in our work on the pentose phosphate pathway ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)