Ch6
... • Compare free energy of reactants, products • Exergonic reactions: reactants have more free energy • Energy is released in reaction ...
... • Compare free energy of reactants, products • Exergonic reactions: reactants have more free energy • Energy is released in reaction ...
Energy Transfer
... Digestion, absorption, and assimilation of relatively large food macromolecules into small subunits. Within the cytosol, AA’s, glucose, fatty acids, and glycerol units are degraded into acetyl-CoA Within the mitochondria, acetyl-CoA degrades to CO2 and H2O with considerable ATP resynthesis. ...
... Digestion, absorption, and assimilation of relatively large food macromolecules into small subunits. Within the cytosol, AA’s, glucose, fatty acids, and glycerol units are degraded into acetyl-CoA Within the mitochondria, acetyl-CoA degrades to CO2 and H2O with considerable ATP resynthesis. ...
Ecology
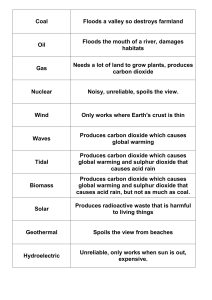
... 2. A pod of dolphins is called a _________________. 3. The Indian River Lagoon is considered an _______________________. 4. A single individual horseshoe crab is called an ___________________. 5. ALL living things are part of the _____________ of Earth. ...
... 2. A pod of dolphins is called a _________________. 3. The Indian River Lagoon is considered an _______________________. 4. A single individual horseshoe crab is called an ___________________. 5. ALL living things are part of the _____________ of Earth. ...
Respiration Webquest
... ATP – The Energy of Life: Go to Biology in Motion. Read and complete the activity. 1. What is ATP? ...
... ATP – The Energy of Life: Go to Biology in Motion. Read and complete the activity. 1. What is ATP? ...
Chapter 16 Citric Acid Cycle
... Several places to feed other compounds into this cycle, and several places to siphon off product for synthesis of other things, so has a central role in metabolism, and has complex control (you thought glycolysis was tough) 16.1 Production of acetate Several different ways compounds can enter the ci ...
... Several places to feed other compounds into this cycle, and several places to siphon off product for synthesis of other things, so has a central role in metabolism, and has complex control (you thought glycolysis was tough) 16.1 Production of acetate Several different ways compounds can enter the ci ...
Cellular Respiration Webquest (word)
... ATP – The Energy of Life: Go to Biology in Motion. Read and complete the activity. 1. What is ATP? ...
... ATP – The Energy of Life: Go to Biology in Motion. Read and complete the activity. 1. What is ATP? ...
Unit 14 ECOSYSTEMS AND COMMUNITIES: ORGANISMS AND
... • Finally, there are specific relationships in which harm does not befall the organisms engaged in the particular interaction. o Define mutualism and give an example. ...
... • Finally, there are specific relationships in which harm does not befall the organisms engaged in the particular interaction. o Define mutualism and give an example. ...
Hein and Arena
... - Ex: palmitic acid, CH3(CH2)14COOH 2) Proteins (amino acids) - Source of reduced carbon atoms that can be catabolized to provide cellular energy. - Provide the major pool of usable nitrogen for cells. ...
... - Ex: palmitic acid, CH3(CH2)14COOH 2) Proteins (amino acids) - Source of reduced carbon atoms that can be catabolized to provide cellular energy. - Provide the major pool of usable nitrogen for cells. ...
Chapter 9 – Respiration
... respiration enable cells to produce ATP without the use of oxygen • Most cellular respiration requires O2 to produce ATP • Without O2, the electron transport chain will cease to ...
... respiration enable cells to produce ATP without the use of oxygen • Most cellular respiration requires O2 to produce ATP • Without O2, the electron transport chain will cease to ...
KATABOLISME KARBOHIDRAT
... The cristae also contain an ATP synthase complex through which hydrogen ions flow down their gradient from the intermembrane space into the matrix. The flow of three H+ through an ATP synthase complex causes a conformational change, which causes the ATP synthase to synthesize ATP from ADP + P. ...
... The cristae also contain an ATP synthase complex through which hydrogen ions flow down their gradient from the intermembrane space into the matrix. The flow of three H+ through an ATP synthase complex causes a conformational change, which causes the ATP synthase to synthesize ATP from ADP + P. ...
Air
... Standard free energy change for oxidation of palmitate to CO2 + H2O = 9800 kJ/mol ATP x 30.5 kJ/mol ...
... Standard free energy change for oxidation of palmitate to CO2 + H2O = 9800 kJ/mol ATP x 30.5 kJ/mol ...
Cellular Respiration Notes (8.3)
... • Final step in the breakdown of glucose Purpose: Converts ADP to ATP by transferring electrons Location: Mitochondria ...
... • Final step in the breakdown of glucose Purpose: Converts ADP to ATP by transferring electrons Location: Mitochondria ...
Cellular Respiration
... Alcoholic fermentation uses pyruvic acid and NADH to produce ATP, alcohol, carbon dioxide, and NAD+. Yeasts and some other microorganisms use alcoholic fermentation. ...
... Alcoholic fermentation uses pyruvic acid and NADH to produce ATP, alcohol, carbon dioxide, and NAD+. Yeasts and some other microorganisms use alcoholic fermentation. ...
chapter 9 cellular respiration: harvesting chemical energy
... kJ) of heat per mole of glucose (about 180 g). This reaction cannot happen at body temperatures. Instead, enzymes within cells lower the barrier of activation energy, allowing sugar to be oxidized in a series of steps. ...
... kJ) of heat per mole of glucose (about 180 g). This reaction cannot happen at body temperatures. Instead, enzymes within cells lower the barrier of activation energy, allowing sugar to be oxidized in a series of steps. ...
4th 9 weeks
... All life is interdependent and interacts with the environment. A rich variety and complexity of organisms have developed in response to changes in the environment. ...
... All life is interdependent and interacts with the environment. A rich variety and complexity of organisms have developed in response to changes in the environment. ...
carbohydrate metabolism
... Catabolism of glucose • The paths that cells use to oxidize glucose completely to carbon dioxide involve many individual chemical reactions. These reactions occur in three different stages. These are: - initial break down of glucose to pyruvate in glycolysis, - further degradation of pyruvate to ac ...
... Catabolism of glucose • The paths that cells use to oxidize glucose completely to carbon dioxide involve many individual chemical reactions. These reactions occur in three different stages. These are: - initial break down of glucose to pyruvate in glycolysis, - further degradation of pyruvate to ac ...
Fermentation - Spencer Community Schools
... Fermenation How do organisms generate energy when oxygen is not available? In the absence of oxygen, fermentation releases energy from food molecules by producing ATP. ...
... Fermenation How do organisms generate energy when oxygen is not available? In the absence of oxygen, fermentation releases energy from food molecules by producing ATP. ...
Nutrients are chemical substances in food that provide energy, form
... of pyruvic acid, Under aerobic conditions, the process of the complete oxidation of glucose continues and pyruvic acid is oxidized to form carbon dioxide and water in two sets of reactions: the Kreb's cycle and the electron transport chain. This is known as aerobic respiration. However, before pyruv ...
... of pyruvic acid, Under aerobic conditions, the process of the complete oxidation of glucose continues and pyruvic acid is oxidized to form carbon dioxide and water in two sets of reactions: the Kreb's cycle and the electron transport chain. This is known as aerobic respiration. However, before pyruv ...
TDH - an Enzyme Involved in Metabolising Threonine to Glycine
... The preliminary structure of L-threonine dehydrogenase has been solved to 2.4Å by X-ray crystallography, which should allow us to hypothesise how this enzyme functions as a biological catalyst at the molecular level. ...
... The preliminary structure of L-threonine dehydrogenase has been solved to 2.4Å by X-ray crystallography, which should allow us to hypothesise how this enzyme functions as a biological catalyst at the molecular level. ...
CH # 9-3
... Fermenation How do organisms generate energy when oxygen is not available? In the absence of oxygen, fermentation releases energy from food molecules by producing ATP. ...
... Fermenation How do organisms generate energy when oxygen is not available? In the absence of oxygen, fermentation releases energy from food molecules by producing ATP. ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)