macromolecules
... The Role of Carbon in Organisms: • Carbon compounds that come from living organisms are called organic compounds. • Two carbon atoms can form various types of covalent bonds—single, double or triple. ...
... The Role of Carbon in Organisms: • Carbon compounds that come from living organisms are called organic compounds. • Two carbon atoms can form various types of covalent bonds—single, double or triple. ...
Plant Cells and Tissues
... Enzymes: catalysts - most of the proteins - proteases Ex. Alpha - amylase ...
... Enzymes: catalysts - most of the proteins - proteases Ex. Alpha - amylase ...
File
... SECTION 1 CARBON COMPOUNDS Objectives Distinguish between organic and inorganic compounds Explain the importance of carbon bonding in biological molecules Identify functional groups in biological molecules Describe how the breaking down of ATP supplies energy to drive chemical reactions ...
... SECTION 1 CARBON COMPOUNDS Objectives Distinguish between organic and inorganic compounds Explain the importance of carbon bonding in biological molecules Identify functional groups in biological molecules Describe how the breaking down of ATP supplies energy to drive chemical reactions ...
AP Macromolecule Notes 09
... o 5 & 6 sided ring structure o Most end in –ose o Hydroxyl –OH & Carbonyl C=O groups o Range from simple to complex, Almost all are hydrophilic ...
... o 5 & 6 sided ring structure o Most end in –ose o Hydroxyl –OH & Carbonyl C=O groups o Range from simple to complex, Almost all are hydrophilic ...
11.2-BIO-CHEM-QUIZ-enzymes-MC
... 2. Which of these correctly matches the molecule with its function? A. lipid—stores genetic information B. vitamin—supplies energy to cells C. enzyme—speeds up chemical reactions D. carbohydrate—manufactures cell membranes 3. Plants such as the Venus Flytrap and the Sun Dew attract and consume insec ...
... 2. Which of these correctly matches the molecule with its function? A. lipid—stores genetic information B. vitamin—supplies energy to cells C. enzyme—speeds up chemical reactions D. carbohydrate—manufactures cell membranes 3. Plants such as the Venus Flytrap and the Sun Dew attract and consume insec ...
CP Physical Science Date :10/18/07
... Amino Acids can Bond to Each Other one at a time, forming a long chain called a POLLYPEPTIDE. Proteins are compose of one or more polypeptides. Some proteins are very large molecules, containing hundreds of Amino Acids. ...
... Amino Acids can Bond to Each Other one at a time, forming a long chain called a POLLYPEPTIDE. Proteins are compose of one or more polypeptides. Some proteins are very large molecules, containing hundreds of Amino Acids. ...
Bacterial Metabolism
... • Reduction and oxidation reaction (LEO goes GER): – Oxidation: loss of electrons, or gain of oxygen, gives increase in oxidation number. – Reduction: gain of electrons, or loss of oxygen, gives decrease in oxidation number. ...
... • Reduction and oxidation reaction (LEO goes GER): – Oxidation: loss of electrons, or gain of oxygen, gives increase in oxidation number. – Reduction: gain of electrons, or loss of oxygen, gives decrease in oxidation number. ...
Download PDF
... structure of proteins, and how this translates into differences in the function of these proteins. We will also cover the synthesis of biopolymers – peptide synthesis from protected amino acids and DNA synthesis from nucleoside phosphoramidites. 2. Energy metabolism. Biological systems use sugars an ...
... structure of proteins, and how this translates into differences in the function of these proteins. We will also cover the synthesis of biopolymers – peptide synthesis from protected amino acids and DNA synthesis from nucleoside phosphoramidites. 2. Energy metabolism. Biological systems use sugars an ...
C383 Study Guide for the Final Exam Spring 2016 Basic Information
... molecule that you store in your liver. Circle the pathways/cycles below that are part of this overall transformation. Cross out any that are not. Gluconeogenesis, pentose phosphate pathway, glycogen synthesis, glycolysis, citric acid cycle B. Trace the metabolic path of this glutamate molecule throu ...
... molecule that you store in your liver. Circle the pathways/cycles below that are part of this overall transformation. Cross out any that are not. Gluconeogenesis, pentose phosphate pathway, glycogen synthesis, glycolysis, citric acid cycle B. Trace the metabolic path of this glutamate molecule throu ...
I Have, Who Has_Photosynthesis_CellResp
... Who has an organism that must get energy from food? I have cellular respiration. Who has a portable form of energy “currency” inside cells? I have pigments. Who has the primary pigment involved in photosynthesis? I have carotenoids. Who has disk-shaped structures found within chloroplasts that conta ...
... Who has an organism that must get energy from food? I have cellular respiration. Who has a portable form of energy “currency” inside cells? I have pigments. Who has the primary pigment involved in photosynthesis? I have carotenoids. Who has disk-shaped structures found within chloroplasts that conta ...
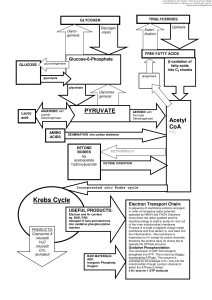
Fermentation and Cellular Respiration 1. Define: Glycolysis
... Glycolysis – Glycolysis is a metabolic pathway allowing for the partial catabolism of glucose. During glycolysis, each glucose molecule is split into two pyruvic acid molecules with the associated production of two molecules of ATP and the reduction of two molecules of NAD to form NADH + H+ (also kn ...
... Glycolysis – Glycolysis is a metabolic pathway allowing for the partial catabolism of glucose. During glycolysis, each glucose molecule is split into two pyruvic acid molecules with the associated production of two molecules of ATP and the reduction of two molecules of NAD to form NADH + H+ (also kn ...
Ch 3
... Fructose is a structural isomer of glucose Galactose is a stereoisomer of glucose Enzymes that act on different sugars can distinguish structural and stereoisomers of this basic six-carbon skeleton Disaccharides • 2 monosaccharides linked together by dehydration synthesis • Used for sugar transport ...
... Fructose is a structural isomer of glucose Galactose is a stereoisomer of glucose Enzymes that act on different sugars can distinguish structural and stereoisomers of this basic six-carbon skeleton Disaccharides • 2 monosaccharides linked together by dehydration synthesis • Used for sugar transport ...
Biochemistry Jeopardy
... hard structures called plaques. Over time, these plaques can block the arteries and cause symptoms and problems throughout the body. Over time, these plaques can block the arteries and cause symptoms and problems throughout the body. ...
... hard structures called plaques. Over time, these plaques can block the arteries and cause symptoms and problems throughout the body. Over time, these plaques can block the arteries and cause symptoms and problems throughout the body. ...
1516 what-is-life-1516
... (like DNA, carbohydrates, proteins, lipids, fats, and oils) 6. Use energy for metabolism ...
... (like DNA, carbohydrates, proteins, lipids, fats, and oils) 6. Use energy for metabolism ...
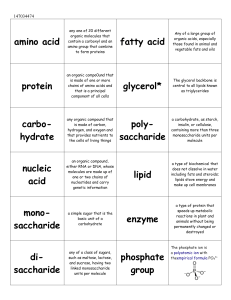
Organic Molecules
... b. Frequently formed with covalent bonds. c. Found in living organisms. d. Usually larger than inorganic molecules (eg: proteins, carbohydrates, lipids, nucleic acids, ATP). e. Many organic molecules are formed by dehydration synthesis (ie: remove H+ from one molecule and OH- from another ...
... b. Frequently formed with covalent bonds. c. Found in living organisms. d. Usually larger than inorganic molecules (eg: proteins, carbohydrates, lipids, nucleic acids, ATP). e. Many organic molecules are formed by dehydration synthesis (ie: remove H+ from one molecule and OH- from another ...
Document
... of very few elements. The six most common are C, H, N, O, P, S. 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activa ...
... of very few elements. The six most common are C, H, N, O, P, S. 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, and nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activa ...
Carbohydrates
... Macromolecule made of lipids and proteins Hydrophilic allows fats to be sheilded from the ...
... Macromolecule made of lipids and proteins Hydrophilic allows fats to be sheilded from the ...
CHE 4310 Fall 2011
... 2. Show the three reactions in the citric acid cycle in which NADH is produced, including the structures. None of these reactions involves molecular oxygen (O2), but all three reactions are strongly inhibited by anaerobic conditions; explain why. ...
... 2. Show the three reactions in the citric acid cycle in which NADH is produced, including the structures. None of these reactions involves molecular oxygen (O2), but all three reactions are strongly inhibited by anaerobic conditions; explain why. ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.