cellular respiration
... • About 40 per cent of the chemical energy present in glucose is transferred to ATP and the remaining 60 per cent appears as heat energy. • The heat energy produced by living cells cannot be used to drive energyrequiring activities, such as muscle contraction or transport against a concentration gra ...
... • About 40 per cent of the chemical energy present in glucose is transferred to ATP and the remaining 60 per cent appears as heat energy. • The heat energy produced by living cells cannot be used to drive energyrequiring activities, such as muscle contraction or transport against a concentration gra ...
CHAPTER 2 VOCABULARY (Highlighted)
... Hydrocarbon chain often bonded to glycerol in a lipid Nonpolar molecule composed of carbon, hydrogen, and oxygen; includes fats and oils. Molecular subunit of a polymer Polymer of nucleotides; the genetic material of organisms. Large, carbon-based molecule formed by monomers. Polymer composed of ami ...
... Hydrocarbon chain often bonded to glycerol in a lipid Nonpolar molecule composed of carbon, hydrogen, and oxygen; includes fats and oils. Molecular subunit of a polymer Polymer of nucleotides; the genetic material of organisms. Large, carbon-based molecule formed by monomers. Polymer composed of ami ...
131110 COS ATP - Community of Reason
... Energy is defined as the capacity to do work, i.e. to move matter. Cells, tissues and organisms need energy for a variety of processes ...
... Energy is defined as the capacity to do work, i.e. to move matter. Cells, tissues and organisms need energy for a variety of processes ...
Biomolecules
... The characteristic pH at which the net electric charge is zero is called the Isoelectric point or “pI”. The amino acid at the isoelectric pI is called “ Zwitter Ion “ and is electrically neutral not migrating in an electric field “Zwitter in German means hybrid or hermaphrodite”. ...
... The characteristic pH at which the net electric charge is zero is called the Isoelectric point or “pI”. The amino acid at the isoelectric pI is called “ Zwitter Ion “ and is electrically neutral not migrating in an electric field “Zwitter in German means hybrid or hermaphrodite”. ...
Chemistry of Life – Macromolecules and Enzymes
... SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. SC.912.L.18.11 Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors, such as pH and temperature, and ...
... SC.912.L.18.1 Describe the basic molecular structures and primary functions of the four major categories of biological macromolecules. SC.912.L.18.11 Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors, such as pH and temperature, and ...
1 - Madison County Schools
... 32. How does a glucose molecule cross the cell membrane? How about ions? Both cross with the assistance of transport proteins. 33. Why were phospholipids so critical in the formation of the first cells? Because they are able to spontaneously self-assemble into simple membranes, providing a "containe ...
... 32. How does a glucose molecule cross the cell membrane? How about ions? Both cross with the assistance of transport proteins. 33. Why were phospholipids so critical in the formation of the first cells? Because they are able to spontaneously self-assemble into simple membranes, providing a "containe ...
17 photosynth 2 10 10 05
... cells at higher concentration than in air Bundle Sheath cells not making oxygen, so very little competitor with C3 reactions ...
... cells at higher concentration than in air Bundle Sheath cells not making oxygen, so very little competitor with C3 reactions ...
Name: Assignment: Cell #4: Structure of Cell Membranes Let`s take
... with each other. This ability is especially important when it comes to fighting diseases. Your immune system uses these markers to differentiate between enemy cells, such as invading bacteria, and your own cells. As a result, your immune system does not attack the wrong cells. (8) What is the role o ...
... with each other. This ability is especially important when it comes to fighting diseases. Your immune system uses these markers to differentiate between enemy cells, such as invading bacteria, and your own cells. As a result, your immune system does not attack the wrong cells. (8) What is the role o ...
Ch. 4: ATP and Cellular Respiration
... Energy • Stored in chemical bonds of compounds. • Compounds that store energy: ATP, NADH and FADH2. • When bonds are broken, energy is released. ...
... Energy • Stored in chemical bonds of compounds. • Compounds that store energy: ATP, NADH and FADH2. • When bonds are broken, energy is released. ...
SI Worksheet 10 1. What does coupling reactions mean? The
... 16. Pepsinogen is the active form of Pepsin, what is the importance and the mechanism that activates it from its inactive form to its active form? HCl cleaves off 42 amino acids, thus, revealing the active site… now it is no longer a zymogen and allowed to break down proteins in the stomach 17. Comp ...
... 16. Pepsinogen is the active form of Pepsin, what is the importance and the mechanism that activates it from its inactive form to its active form? HCl cleaves off 42 amino acids, thus, revealing the active site… now it is no longer a zymogen and allowed to break down proteins in the stomach 17. Comp ...
SADDLEBACK COLLEGE BIOLOGY 20 EXAMINATION 2 STUDY
... • Know the two laws of thermodynamics (Which laws are known as the conservation of energy?) • What is metabolism? Catabolism? Anabolism? • ATP - how it works • What are enzymes and how they work? Chapter 5 • what is an active site - what types of molecules bind there • know the factors that influenc ...
... • Know the two laws of thermodynamics (Which laws are known as the conservation of energy?) • What is metabolism? Catabolism? Anabolism? • ATP - how it works • What are enzymes and how they work? Chapter 5 • what is an active site - what types of molecules bind there • know the factors that influenc ...
A1984TR03900001
... of the membranes that permit differential uptake and release of metabolites from particular organdIes are currently under intense investigation. Solutions to problems of membrane selectivity will help to define the actual role of compartmentation in the regulation of metabolism. “In the review, we s ...
... of the membranes that permit differential uptake and release of metabolites from particular organdIes are currently under intense investigation. Solutions to problems of membrane selectivity will help to define the actual role of compartmentation in the regulation of metabolism. “In the review, we s ...
The Chemical Level of Organization
... include water and mineral salts that dissolve (and ionize) in water. Organic compounds are based on a backbone of carbon atoms, with nitrogen, oxygen, hydrogen, and other elements contributing to the structure as well. Biologically important classes of organic compounds include carbohydrates, lipids ...
... include water and mineral salts that dissolve (and ionize) in water. Organic compounds are based on a backbone of carbon atoms, with nitrogen, oxygen, hydrogen, and other elements contributing to the structure as well. Biologically important classes of organic compounds include carbohydrates, lipids ...
Cellular Respiration
... NADH and FADH2 release electrons and their H+ ions This turns them into NAD+ and FAD H+ ions are sequestered in the inner mitochondrial space H+ ions diffuse down their concentration gradient through ATP synthase Oxygen is the final electron acceptor molecule in the ETC The maximum amount of ATP pro ...
... NADH and FADH2 release electrons and their H+ ions This turns them into NAD+ and FAD H+ ions are sequestered in the inner mitochondrial space H+ ions diffuse down their concentration gradient through ATP synthase Oxygen is the final electron acceptor molecule in the ETC The maximum amount of ATP pro ...
008 Chapter 08 Metabolism: Energy Enzymes and Regulation 1
... 37. An organism may use glycolysis and the pentose phosphate pathway simultaneously. True False 38. In addition to being used in the making of ATP, proton motive force is used directly to power the rotation of bacterial flagella. True False 39. Although most metabolic reactions are freely reversible ...
... 37. An organism may use glycolysis and the pentose phosphate pathway simultaneously. True False 38. In addition to being used in the making of ATP, proton motive force is used directly to power the rotation of bacterial flagella. True False 39. Although most metabolic reactions are freely reversible ...
Chapter 2 Biochemistry Goux Guided Notes
... - Strong forces bind ____protons____and ___nuetrons__________together to form the nucleus, which is the center of the atom - The __electron_________is a negatively charged particle - Electrons are in constant motion in the space surrounding the nucleus. They are attracted to the positively charged n ...
... - Strong forces bind ____protons____and ___nuetrons__________together to form the nucleus, which is the center of the atom - The __electron_________is a negatively charged particle - Electrons are in constant motion in the space surrounding the nucleus. They are attracted to the positively charged n ...
ENERGY
... Radiant: sunlight, EM waves Chemical: Glucose, ATP, Starch Kinetic: Molecular movement (diffusion, osmosis) Heat Mechanical: Muscle contraction ...
... Radiant: sunlight, EM waves Chemical: Glucose, ATP, Starch Kinetic: Molecular movement (diffusion, osmosis) Heat Mechanical: Muscle contraction ...
The Electron Transport Chain Chemiosmosis
... approximately 277.4 kcal of energy. If all of this energy is released at one time, then most of it would be lost as heat. Burning the energy all at once would be akin to igniting your gas tank in order to run your car, rather than burning small amounts of gasoline slowly in the engine. If the energy ...
... approximately 277.4 kcal of energy. If all of this energy is released at one time, then most of it would be lost as heat. Burning the energy all at once would be akin to igniting your gas tank in order to run your car, rather than burning small amounts of gasoline slowly in the engine. If the energy ...
Understanding Our Environment
... (PS I) then photosystem II (PS II) and finally to NADP to produce NADPH. H+ are used to produce ATP via ATP synthase. Oxygen gas, produced from splitting water, is a byproduct of noncyclic photophosphorylation. Cyclic photophosphorylation: Only PS I is involved. Electrons boosted from PS I are shunt ...
... (PS I) then photosystem II (PS II) and finally to NADP to produce NADPH. H+ are used to produce ATP via ATP synthase. Oxygen gas, produced from splitting water, is a byproduct of noncyclic photophosphorylation. Cyclic photophosphorylation: Only PS I is involved. Electrons boosted from PS I are shunt ...
Prentice hall Biology Worksheets
... Identifying On the lines provided, identify each statement as describing carbohydrates, lipids, nucleic acids, or proteins. 1. the main source of energy for living things 2. help carry out chemical reactions 3. important parts of biological membranes 4. contain hydrogen, oxygen, nitrogen, phosphorus ...
... Identifying On the lines provided, identify each statement as describing carbohydrates, lipids, nucleic acids, or proteins. 1. the main source of energy for living things 2. help carry out chemical reactions 3. important parts of biological membranes 4. contain hydrogen, oxygen, nitrogen, phosphorus ...
Biotechnology Unit 3: DNA to Proteins Essential Cell Biology
... a. They can range in size from approximately 30 amino acids to more than 10,000 but most are between 50 and 2,000 amino acids b. They can be globular, fibrous, filamentous, sheets, rings, spheres, and many other shapes The shape of a protein is specified by its amino acid sequence a. There are 20 di ...
... a. They can range in size from approximately 30 amino acids to more than 10,000 but most are between 50 and 2,000 amino acids b. They can be globular, fibrous, filamentous, sheets, rings, spheres, and many other shapes The shape of a protein is specified by its amino acid sequence a. There are 20 di ...
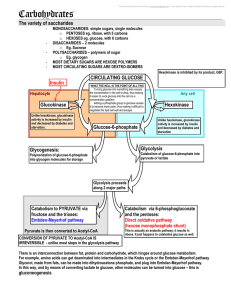
5 carbohydrates and the Krebs Cycle
... the whole point is to convert Acetyl-CoA to CO2 and hydrogen. Acetyl-CoA is the major entry point, but amino acids when deaminated can enter at various points along the cycle This cycle requires O2 and does not function under anaerobic conditions ...
... the whole point is to convert Acetyl-CoA to CO2 and hydrogen. Acetyl-CoA is the major entry point, but amino acids when deaminated can enter at various points along the cycle This cycle requires O2 and does not function under anaerobic conditions ...
Chem 400 Biochemistry I
... •Biochemistry is essential to all of the life sciences (biomedical and plant sciences) All advanced degrees require that biochemistry is one of the first courses •This class will be taught not - as an advanced organic but as an encompassing science that should help tie several of your classes togeth ...
... •Biochemistry is essential to all of the life sciences (biomedical and plant sciences) All advanced degrees require that biochemistry is one of the first courses •This class will be taught not - as an advanced organic but as an encompassing science that should help tie several of your classes togeth ...
Dehydration Synthesis 2.cwk
... Dehydration Synthesis Activity For each section, you will be simulation the dehydration synthesis reaction. In other words, you will be removing water in order to join together two (or more) molecules. Use your book and notes to get an idea of the building block molecules you are using (sugars, glyc ...
... Dehydration Synthesis Activity For each section, you will be simulation the dehydration synthesis reaction. In other words, you will be removing water in order to join together two (or more) molecules. Use your book and notes to get an idea of the building block molecules you are using (sugars, glyc ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.