Photsynthesis III - Light Indpendent
... – 3 Carbon Dioxides enter the cycle and combine with RuBP to create 3 very unstable 6 carbon molecules. – These 6-carbon molecules instantly break down into 6, 3-carbon molecules that are much more stable. – We now have all of the carbons in the cycle that are needed to make ½ of a glucose molecule. ...
... – 3 Carbon Dioxides enter the cycle and combine with RuBP to create 3 very unstable 6 carbon molecules. – These 6-carbon molecules instantly break down into 6, 3-carbon molecules that are much more stable. – We now have all of the carbons in the cycle that are needed to make ½ of a glucose molecule. ...
Bioenergetics and Metabolism
... 4. What are examples of glycolysis in real life? Glycolysis is the sole source of ATP under anaerobic conditions which can occur in animal muscle tissue during intense exercise. Fermentation also relies on glycolysis which is a process that is used to make alcoholic beverages when yeast cells are pr ...
... 4. What are examples of glycolysis in real life? Glycolysis is the sole source of ATP under anaerobic conditions which can occur in animal muscle tissue during intense exercise. Fermentation also relies on glycolysis which is a process that is used to make alcoholic beverages when yeast cells are pr ...
File - Mr. Shanks` Class
... carbons into a series of acetyl-CoA The oxidation of fatty acids into acetyl-CoA molecules requires the breaking of bonds, always one less bond that the number of acetyl-CoA. To break bonds, we must add water and ATP. When these fatty acid bonds are broken, 1 FADH2 and 1 [NADH + H+] are produced. ...
... carbons into a series of acetyl-CoA The oxidation of fatty acids into acetyl-CoA molecules requires the breaking of bonds, always one less bond that the number of acetyl-CoA. To break bonds, we must add water and ATP. When these fatty acid bonds are broken, 1 FADH2 and 1 [NADH + H+] are produced. ...
Practice Exam 3 Answers
... Name the two enzymes that catalyze a reaction in which ATP is consumed? __________________________________________ Which enzyme catalyzes a reaction in which NADH is produced? _____________________ Which enzyme converts G3P into 1,3 BPG? __________________________ Name two enzyme reactions from glyc ...
... Name the two enzymes that catalyze a reaction in which ATP is consumed? __________________________________________ Which enzyme catalyzes a reaction in which NADH is produced? _____________________ Which enzyme converts G3P into 1,3 BPG? __________________________ Name two enzyme reactions from glyc ...
2. Microbial Growth Kinetics
... observed transfer to fresh medium so that stabilization and subsequent purification is performed in a continuous culture Periodic inoculation of soil or sewage to the culture will ensure as the source of potential isolates; dominants must be resistant to ...
... observed transfer to fresh medium so that stabilization and subsequent purification is performed in a continuous culture Periodic inoculation of soil or sewage to the culture will ensure as the source of potential isolates; dominants must be resistant to ...
Homework 7 - Fullfrontalanatomy.com
... generation of ATP by chemiosmosis; d) pumping of hydrogens into the inner cristae space for later generation of ATP by chemiosmosis; e) preparation of water for eventual incorporation into glucose 26. ATP is known as the energy currency of the cell because ____. a) ATP is the most readily usable for ...
... generation of ATP by chemiosmosis; d) pumping of hydrogens into the inner cristae space for later generation of ATP by chemiosmosis; e) preparation of water for eventual incorporation into glucose 26. ATP is known as the energy currency of the cell because ____. a) ATP is the most readily usable for ...
BIOMOLECULES : CARBOHYDRATES - IDC
... have ever "counted" your carbs, you know that one biological function of CHOs is to store and, on oxidation, provide energy to the body for required functions. Instead of concentrating on how CHOs are used for energy production, we will focus predominantly on their structures, which allows them to e ...
... have ever "counted" your carbs, you know that one biological function of CHOs is to store and, on oxidation, provide energy to the body for required functions. Instead of concentrating on how CHOs are used for energy production, we will focus predominantly on their structures, which allows them to e ...
What is Biology?
... • Autotrophs – transform energy from their environment (the “producers”) – Plants are autotrophs; they transform the sun’s energy into energy-rich molecules that support life • Heterotrophs – ingest their energy from their environment (the “consumers”) – Animals are heterotrophs; they ingest (eat) f ...
... • Autotrophs – transform energy from their environment (the “producers”) – Plants are autotrophs; they transform the sun’s energy into energy-rich molecules that support life • Heterotrophs – ingest their energy from their environment (the “consumers”) – Animals are heterotrophs; they ingest (eat) f ...
What is Biology? - sunysuffolk.edu
... • Autotrophs – transform energy from their environment (the “producers”) – Plants are autotrophs; they transform the sun’s energy into energy-rich molecules that support life • Heterotrophs – ingest their energy from their environment (the “consumers”) – Animals are heterotrophs; they ingest (eat) f ...
... • Autotrophs – transform energy from their environment (the “producers”) – Plants are autotrophs; they transform the sun’s energy into energy-rich molecules that support life • Heterotrophs – ingest their energy from their environment (the “consumers”) – Animals are heterotrophs; they ingest (eat) f ...
Biology 2107/03
... conversion of glucose into pyruvic acid conversion of pyruvic acid into lactic acid conversion of pyruvic acid into acetyl CoA and CO2 citric acid (Kreb’s) cycle respiratory electron transport chain ...
... conversion of glucose into pyruvic acid conversion of pyruvic acid into lactic acid conversion of pyruvic acid into acetyl CoA and CO2 citric acid (Kreb’s) cycle respiratory electron transport chain ...
The amino acids
... Di-peptide Amino acids bind, to form a protein. Upon binding, two protons from the NH3 and one oxygen from the carboxyl join to form a water. So the peptide bond has at the one side a C=O and at the other side an N-H. Only the ends of the chain are NH3 or carboxylic. ...
... Di-peptide Amino acids bind, to form a protein. Upon binding, two protons from the NH3 and one oxygen from the carboxyl join to form a water. So the peptide bond has at the one side a C=O and at the other side an N-H. Only the ends of the chain are NH3 or carboxylic. ...
103 final review worksheet
... 94. Draw a diagram of ATP synthase, showing the two subunits, F0 an F1. Show where the protein crosses the intermitochondrial membrane and label the intermembrane space and the matrix. Show where the protons enter, where they leave and where the ATP is synthesized. ...
... 94. Draw a diagram of ATP synthase, showing the two subunits, F0 an F1. Show where the protein crosses the intermitochondrial membrane and label the intermembrane space and the matrix. Show where the protons enter, where they leave and where the ATP is synthesized. ...
Due: 2015. 10. 12. 11:00 am (월)
... The kinetics of allosteric enzymes usually does not fit on Michaelis-Menten equation because modulator (regulator) that binds to the enzyme changes the activity on the substrate(S). Thus there are two states, R and T state. A model that hypothesizes the existence of equilibrium between the two state ...
... The kinetics of allosteric enzymes usually does not fit on Michaelis-Menten equation because modulator (regulator) that binds to the enzyme changes the activity on the substrate(S). Thus there are two states, R and T state. A model that hypothesizes the existence of equilibrium between the two state ...
- University of Duhok
... This course covers topics such as bonding and structures, acids and bases, organic molecules and functional groups, alkanes, stereochemistry, organic reactions, oxidation and reduction; solutions, colloids; emulsions; and other various topics. This course also give fundamental concepts relating to c ...
... This course covers topics such as bonding and structures, acids and bases, organic molecules and functional groups, alkanes, stereochemistry, organic reactions, oxidation and reduction; solutions, colloids; emulsions; and other various topics. This course also give fundamental concepts relating to c ...
Protein Synthesis
... check it. If there are any errors, please go back and find your mistakes. 8. Did you have any “mutations” during the process? ____________ ...
... check it. If there are any errors, please go back and find your mistakes. 8. Did you have any “mutations” during the process? ____________ ...
External sources of energy → biologically energy : ATP
... C6H12O6 + 2NAD+ + 2ADP3- + 2Pi2- 2 C3H4O3 + 2NADH + 2 ATP4• Citric acid cycle • In mitochondrion • Pyruvate CO2 + NADH + FADH2 • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
... C6H12O6 + 2NAD+ + 2ADP3- + 2Pi2- 2 C3H4O3 + 2NADH + 2 ATP4• Citric acid cycle • In mitochondrion • Pyruvate CO2 + NADH + FADH2 • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
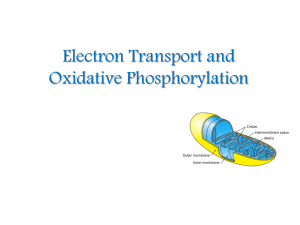
Electron Transport and Oxidative Phosphorylation
... The inner mitochondrial membrane contains 5 separate enzyme complexes, called compelexes I, II, III, IV and V. Each complex accepts or donates electrons to mobile carrier, such as coenzyme Q and cytochrome c. The electrons ultimately combine with oxygen and protons to form water. ...
... The inner mitochondrial membrane contains 5 separate enzyme complexes, called compelexes I, II, III, IV and V. Each complex accepts or donates electrons to mobile carrier, such as coenzyme Q and cytochrome c. The electrons ultimately combine with oxygen and protons to form water. ...
File
... o Products: substances made after a chemical reaction. o Metabolism is the sum total of all chemical reactions in a cell. ...
... o Products: substances made after a chemical reaction. o Metabolism is the sum total of all chemical reactions in a cell. ...
(key)
... 6. For integral proteins associated with cell membranes what type of amino acid residues would be found exclusively within the membrane. l\)0'1\ - ...
... 6. For integral proteins associated with cell membranes what type of amino acid residues would be found exclusively within the membrane. l\)0'1\ - ...
Objectives Chapter 6 - Mercer County Community College
... 2 ATP per 1 glucose (2 pyruvate) CO2 generated NADH and FADH2 (electron donors) Pyruvate converted to acetyl CoA prior to cycle Oxidative phosphorylation Occurs in mitochondrial cristae NADH and FADH2 donate electrons to electron transport chain Cytochrome proteins involved Oxygen required H+ gradie ...
... 2 ATP per 1 glucose (2 pyruvate) CO2 generated NADH and FADH2 (electron donors) Pyruvate converted to acetyl CoA prior to cycle Oxidative phosphorylation Occurs in mitochondrial cristae NADH and FADH2 donate electrons to electron transport chain Cytochrome proteins involved Oxygen required H+ gradie ...
Fatty Acid and Phospholipid Class Activity 1. Draw the skeletal
... The distance between the two highlighted carbons on cholesterol is almost exactly 10 Å (angstroms where 1 Å = 10-10 m)). Noting that the distance between C1 and C3 in propane is 251 pm, predict how the position of the 1st double relative to the carboxylic acid. For example ∆3 would be the 3rd carbon ...
... The distance between the two highlighted carbons on cholesterol is almost exactly 10 Å (angstroms where 1 Å = 10-10 m)). Noting that the distance between C1 and C3 in propane is 251 pm, predict how the position of the 1st double relative to the carboxylic acid. For example ∆3 would be the 3rd carbon ...
Activity 4.1/5.1 How can you identify organic macromolecules?
... Predict where you would find each amino acid: in the interior portion of the protein (away from water) or on the outside of the protein (facing water). (Refer to Figure 5.16, ...
... Predict where you would find each amino acid: in the interior portion of the protein (away from water) or on the outside of the protein (facing water). (Refer to Figure 5.16, ...
Classification of Enzymes - Lectures For UG-5
... “a” is the class, “b” is the subclass, “c” is the subsubclass, and “d” is the sub-sub-subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Example: for Alcoho ...
... “a” is the class, “b” is the subclass, “c” is the subsubclass, and “d” is the sub-sub-subclass. The “b” and “c” digits describe the reaction, while the “d” digit is used to distinguish between different enzymes of the same function based on the actual substrate in the reaction. • Example: for Alcoho ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.