acetyl CoA + HCO3
... How do phospholipids in the outer leaflet of the plasma membrane get there? ...
... How do phospholipids in the outer leaflet of the plasma membrane get there? ...
Notes-Cellular Respiration
... 1.A)Explain where organisms get the energy needed for life processes. • Organisms get the energy they need from food. • Energy stored in food is expressed as calories. • Calorie • amount of energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. • 1000 calories = 1 kilocalori ...
... 1.A)Explain where organisms get the energy needed for life processes. • Organisms get the energy they need from food. • Energy stored in food is expressed as calories. • Calorie • amount of energy needed to raise the temperature of 1 gram of water by 1 degree Celsius. • 1000 calories = 1 kilocalori ...
Metabolism of lipids
... Electrons reach CoQ via Complexes I and II. CoQH2 serves as a mobile carrier of electrons and protons. It transfers electrons to Complex III, which transfers them to another mobile connecting link, cytochrome c. Complex IV transfers electrons from reduced cytochrome c to O2. Electron flow through Co ...
... Electrons reach CoQ via Complexes I and II. CoQH2 serves as a mobile carrier of electrons and protons. It transfers electrons to Complex III, which transfers them to another mobile connecting link, cytochrome c. Complex IV transfers electrons from reduced cytochrome c to O2. Electron flow through Co ...
Bioenergetics Free Energy Change
... ATP to phosphorylate compounds ranked lower than itself. It is favorable to synthesize ATP from ADP by higher ranked PEP and CP. • It is harder to synthesize the highest ranking molecules and the lowest ranking ones don’t release enough energy to perform significant work. Thus, ATP’s intermediate ra ...
... ATP to phosphorylate compounds ranked lower than itself. It is favorable to synthesize ATP from ADP by higher ranked PEP and CP. • It is harder to synthesize the highest ranking molecules and the lowest ranking ones don’t release enough energy to perform significant work. Thus, ATP’s intermediate ra ...
a ANSWER - Cornerstone Charter Academy
... of the different entry pathways to cellular respiration when different macromolecules are digested for energy production. Why are none of the digestive products entering the electron transport chain, directly? ...
... of the different entry pathways to cellular respiration when different macromolecules are digested for energy production. Why are none of the digestive products entering the electron transport chain, directly? ...
Characteristics of Living Things (Essay
... Organic Molecules: what elements? Subcomponents? Condensation & hydrolysis reactions? I. Lipids: energy storage phospholipids vs. fats vs. fatty acids Steroids: big molecules with carbon rings Differences vs. similarities II. Carbohydrates - monosaccharides vs. disaccharides vs. polysaccharides -glu ...
... Organic Molecules: what elements? Subcomponents? Condensation & hydrolysis reactions? I. Lipids: energy storage phospholipids vs. fats vs. fatty acids Steroids: big molecules with carbon rings Differences vs. similarities II. Carbohydrates - monosaccharides vs. disaccharides vs. polysaccharides -glu ...
OH - H + - WordPress.com
... roots of a plant to the leaves so that photosynthesis can occur. Transpiration (water evaporating from the leaves of plants) drives the capillary action; as one water molecule evaporates, the next water molecule is pulled up. Capillary action also allows blood flow throughout animal tissues. ...
... roots of a plant to the leaves so that photosynthesis can occur. Transpiration (water evaporating from the leaves of plants) drives the capillary action; as one water molecule evaporates, the next water molecule is pulled up. Capillary action also allows blood flow throughout animal tissues. ...
QUEST Study guide Organic molecules Proteins, carbohydrates
... Organic molecules Proteins, carbohydrates, lipids, & nucleic acids (just know that these are DNA & RNA) Notes & power point on website Know the biological functions of ALL these molecules Know how to test for glucose, starch, protein, lipids ...
... Organic molecules Proteins, carbohydrates, lipids, & nucleic acids (just know that these are DNA & RNA) Notes & power point on website Know the biological functions of ALL these molecules Know how to test for glucose, starch, protein, lipids ...
ATP
... Formation of , sugar / glucose / hexose / sucrose / starch / cellulose Formation of fat / triglyceride / lipid fatty acids / glycerol / amino acids / protein / nucleic acids / nucleotides Most triose phosphate used to produce RuBP and the rest for production of hexose ...
... Formation of , sugar / glucose / hexose / sucrose / starch / cellulose Formation of fat / triglyceride / lipid fatty acids / glycerol / amino acids / protein / nucleic acids / nucleotides Most triose phosphate used to produce RuBP and the rest for production of hexose ...
ch 9 Cellular_Respiration
... • NAD+ - nicotinamide adenine dinucleotide is a coenzyme that transports electrons from glucose to the electron transport chain to make ATP • NAD+ is reduced (electrons are added) to NADH + H+ using the enzyme dehydrogenase (2 electrons and 2 protons, but one proton is released) ...
... • NAD+ - nicotinamide adenine dinucleotide is a coenzyme that transports electrons from glucose to the electron transport chain to make ATP • NAD+ is reduced (electrons are added) to NADH + H+ using the enzyme dehydrogenase (2 electrons and 2 protons, but one proton is released) ...
Exam 1 2007 - chem.uwec.edu
... 5. What two 3-carbon molecules are generated by the cleavage of fructose-1,6bisphosphate? A) glyceraldehyde-3-phosphate and 3-phosphoglycerate B) glyceraldehyde-3-phosphate and dihydroxyacetone phosphate C) pyruvate and phosphoenolpyruvate D) enolase and 2-phosphoglycerate E) glyceraldehyde-3-phosph ...
... 5. What two 3-carbon molecules are generated by the cleavage of fructose-1,6bisphosphate? A) glyceraldehyde-3-phosphate and 3-phosphoglycerate B) glyceraldehyde-3-phosphate and dihydroxyacetone phosphate C) pyruvate and phosphoenolpyruvate D) enolase and 2-phosphoglycerate E) glyceraldehyde-3-phosph ...
ppt
... 1. Structure 2. Functions a. energy storage... but since they probably do other things, these are metabolized last... b. structure - after water, animals are mostly protein collagen, elastin, actin, myosin, etc... ...
... 1. Structure 2. Functions a. energy storage... but since they probably do other things, these are metabolized last... b. structure - after water, animals are mostly protein collagen, elastin, actin, myosin, etc... ...
Origin of Life
... • Hypothesis: Energy from lightning created organic materials from inorganic ingredients • Experimental Set-Up: – Ammonia, H2O vapor, Methane, CO gases added – Electricity added (simulate lightning) ...
... • Hypothesis: Energy from lightning created organic materials from inorganic ingredients • Experimental Set-Up: – Ammonia, H2O vapor, Methane, CO gases added – Electricity added (simulate lightning) ...
coupling membrane
... 4) the oxidation of reduced cofactors by oxygen forming water and releasing energy (respiratory electron transfer) 5) the synthesis of ATP from ADP and phosphate using energy released during electron transfer (oxidative phosphorylation) There is also transamination of amino-acids to produce acetyl c ...
... 4) the oxidation of reduced cofactors by oxygen forming water and releasing energy (respiratory electron transfer) 5) the synthesis of ATP from ADP and phosphate using energy released during electron transfer (oxidative phosphorylation) There is also transamination of amino-acids to produce acetyl c ...
CHAPTER 19 ORIGIN AND HISTORY OF LIFE
... b. Ribozymes are RNA that acts as enzymes. c. Some viruses contain RNA genes with a protein enzyme called reverse transcriptase that uses RNA as a template to form DNA; this could have given rise to the first DNA. 3. The protein-first hypothesis contends that proteins or at least polypeptides were t ...
... b. Ribozymes are RNA that acts as enzymes. c. Some viruses contain RNA genes with a protein enzyme called reverse transcriptase that uses RNA as a template to form DNA; this could have given rise to the first DNA. 3. The protein-first hypothesis contends that proteins or at least polypeptides were t ...
Science Proficiency Words - Group 1
... 9. The conversion of light energy into chemical energy by living organisms. The raw materials are carbon dioxide and water, the energy source is sunlight, and the end products include glucose and oxygen. 10. Deoxyribonucleic acid (DNA) is a nucleic acid that contains the genetic instructions used in ...
... 9. The conversion of light energy into chemical energy by living organisms. The raw materials are carbon dioxide and water, the energy source is sunlight, and the end products include glucose and oxygen. 10. Deoxyribonucleic acid (DNA) is a nucleic acid that contains the genetic instructions used in ...
study guide
... Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. Cells store energy by adding a phosphate group to adenosine diphosphate (ADP) molecules. Cells release energy from ATP m ...
... Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. Cells store energy by adding a phosphate group to adenosine diphosphate (ADP) molecules. Cells release energy from ATP m ...
Oxidative phosphorylation
... • Pyruvate converted to ethanol, releasing CO2 • NADH is oxidized to make NAD+ ...
... • Pyruvate converted to ethanol, releasing CO2 • NADH is oxidized to make NAD+ ...
File - Mr. Holz`s Website
... 14. Know that enzymes are the catalysts in living cells. 15. Know the 4 main properties of enzymes: a. They are proteins b. They bind to specific substrates at the ACTIVE SITE like a lock and key c. Enzymes remain unchanged after a reaction, so they can continue doing their job (1 enzyme can bind to ...
... 14. Know that enzymes are the catalysts in living cells. 15. Know the 4 main properties of enzymes: a. They are proteins b. They bind to specific substrates at the ACTIVE SITE like a lock and key c. Enzymes remain unchanged after a reaction, so they can continue doing their job (1 enzyme can bind to ...
B4 revision ppt
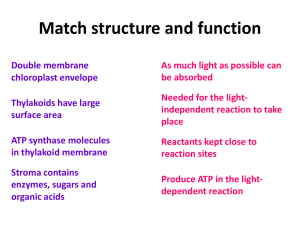
... Photosynthesis takes place in chloroplasts. Contain chlorophyll which absorbs light and uses the energy to start photosynthesis Energy from light splits water molecules into H2 and O2 atoms. The H2 is combined with CO2 from the air to make glucose. O2 is released as a waste product ...
... Photosynthesis takes place in chloroplasts. Contain chlorophyll which absorbs light and uses the energy to start photosynthesis Energy from light splits water molecules into H2 and O2 atoms. The H2 is combined with CO2 from the air to make glucose. O2 is released as a waste product ...
Practice Exam1
... B. Yes, if it is coupled to another reaction. C. Yes, it is spontaneous. D. No, it will never occur. E. Yes, if it takes place within a constrained area. 9. The type of structure to which α-helices, β sheets, and turns are referred. A. Primary structure B. Secondary structure C. Tertiary structure D ...
... B. Yes, if it is coupled to another reaction. C. Yes, it is spontaneous. D. No, it will never occur. E. Yes, if it takes place within a constrained area. 9. The type of structure to which α-helices, β sheets, and turns are referred. A. Primary structure B. Secondary structure C. Tertiary structure D ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.