Name: #: Cellular Respiration Review 2 Process Where does it
... 7. Why do we say there is a ‘net’ gain of 2 ATP at the end of glycolysis? Glycolysis produces 4ATP but since it needs 2 ATP to start, the cell only increases its amount of ATP by 2 ...
... 7. Why do we say there is a ‘net’ gain of 2 ATP at the end of glycolysis? Glycolysis produces 4ATP but since it needs 2 ATP to start, the cell only increases its amount of ATP by 2 ...
Properties of Water
... acids – fatty acids, amino acids D. Amino groups – NH2 - form amines – amino acids E. Sulfhydryl groups – SH - forms thiols – cross linking proteins F. Phosphate groups – PO4- form phosphates – important part of nucleotides and ATP G. Methyl groups – CH3 – important for ...
... acids – fatty acids, amino acids D. Amino groups – NH2 - form amines – amino acids E. Sulfhydryl groups – SH - forms thiols – cross linking proteins F. Phosphate groups – PO4- form phosphates – important part of nucleotides and ATP G. Methyl groups – CH3 – important for ...
Unit 2 - OCCC.edu
... Cofactors may be ___________________ (such as a metal in ionic form) or ________________ An organic cofactor is called a _____________________ Coenzymes include ___________________________ Enzyme Inhibitors __________________________________________________ bind to the active site of an enzyme, com ...
... Cofactors may be ___________________ (such as a metal in ionic form) or ________________ An organic cofactor is called a _____________________ Coenzymes include ___________________________ Enzyme Inhibitors __________________________________________________ bind to the active site of an enzyme, com ...
ELECTRON TRANSPORT CHAIN, OXIDATIVE
... Discipline of Biochemistry & Molecular Biology, BMLS II, Bpharm II, BDS II VJ Temple ...
... Discipline of Biochemistry & Molecular Biology, BMLS II, Bpharm II, BDS II VJ Temple ...
anaerobic respiration
... When food is broken down, energetic electrons are released. NADH catches the electrons. NADH releases the electrons so that ATP can be made. Metabolism is all of the reactions in the body that involve energy transformation ...
... When food is broken down, energetic electrons are released. NADH catches the electrons. NADH releases the electrons so that ATP can be made. Metabolism is all of the reactions in the body that involve energy transformation ...
Enzymes
... • Enzymes are catalysts, substances that change the rate of a chemical reaction. • Enzymes are unchanged by the reaction (which is why they can be reused over and over again)! • Enzymes are named after their substrates. – The name for an enzyme generally ends in “ase”. ...
... • Enzymes are catalysts, substances that change the rate of a chemical reaction. • Enzymes are unchanged by the reaction (which is why they can be reused over and over again)! • Enzymes are named after their substrates. – The name for an enzyme generally ends in “ase”. ...
A chemist has discovered a drug that blocks
... How are these 2 reactions similar? 20. In the presence of a metabolic poison that specifically and completely inhibit the function of mitochondrial ATP synthase, how would you expect the pH difference to change across the inner mitochondrial membrane? What would be the ultimate fate of ATP productio ...
... How are these 2 reactions similar? 20. In the presence of a metabolic poison that specifically and completely inhibit the function of mitochondrial ATP synthase, how would you expect the pH difference to change across the inner mitochondrial membrane? What would be the ultimate fate of ATP productio ...
II. Pre-test to identify student misconceptions prior to addressing the
... Glycolysis produces ATP by substrate level phosphorylation. True ...
... Glycolysis produces ATP by substrate level phosphorylation. True ...
Pantothenic Acid - Pure Encapsulations
... What Is The Source? Calcium pantothenate provides pantothenic acid and is synthetic. Ascorbyl palmitate is derived from corn dextrose fermentation and palm oil. Hypo-allergenic plant fiber is ...
... What Is The Source? Calcium pantothenate provides pantothenic acid and is synthetic. Ascorbyl palmitate is derived from corn dextrose fermentation and palm oil. Hypo-allergenic plant fiber is ...
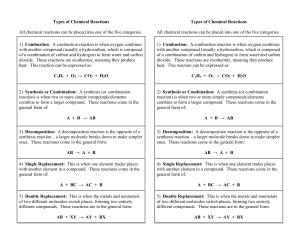
Types of Chemical Reactions
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
Proteins
... Salt linkage-Electrostatic or Ionic linkages found in Proteins Protein data book- Structure of various Proteins is studied using X ray diffraction and stored in data base Methods to separate proteins- Electrophoresis, Chromatography, HPLC, Affinity chromatography London dispersion and Vander Waal fo ...
... Salt linkage-Electrostatic or Ionic linkages found in Proteins Protein data book- Structure of various Proteins is studied using X ray diffraction and stored in data base Methods to separate proteins- Electrophoresis, Chromatography, HPLC, Affinity chromatography London dispersion and Vander Waal fo ...
Secondary Structure of Proteins
... Methanol is toxic – 10 mL can cause blindness due to its metabolite formic acid. Treatment may include alcohol dehydrogenase inhibitor (fomepizole, Antizol®) and ethanol Fomepizole and ethanol both compete with methanol for binding alcohol dehydrogenase. ...
... Methanol is toxic – 10 mL can cause blindness due to its metabolite formic acid. Treatment may include alcohol dehydrogenase inhibitor (fomepizole, Antizol®) and ethanol Fomepizole and ethanol both compete with methanol for binding alcohol dehydrogenase. ...
222-1
... • Link an endogenous solubilizing moiety either to the original drug (if polar function are already present) or to the phase I metabolite. • Common solubilizing groups are glucuronic acid, various amino acids or sulphate groups. • The conjugate molecule, being more polar and water-soluble, is usuall ...
... • Link an endogenous solubilizing moiety either to the original drug (if polar function are already present) or to the phase I metabolite. • Common solubilizing groups are glucuronic acid, various amino acids or sulphate groups. • The conjugate molecule, being more polar and water-soluble, is usuall ...
Cellular Respiration
... splitting of glucose – 2 pyruvate, yield 2 ATP Preparatory reaction – in mitochondria, pyruvate oxidized to 2 – C acetyl group, preps for citric acid cycle Citric acid cycle – (Krebs) in matrix of mitochondria, yield 2 ATP Electron transport chain – cristae, oxygen is final electron acceptor and for ...
... splitting of glucose – 2 pyruvate, yield 2 ATP Preparatory reaction – in mitochondria, pyruvate oxidized to 2 – C acetyl group, preps for citric acid cycle Citric acid cycle – (Krebs) in matrix of mitochondria, yield 2 ATP Electron transport chain – cristae, oxygen is final electron acceptor and for ...
Structure and Function of Macromolecules
... proteins, and nucleic acids—form chainlike molecules called polymers. A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds. The repeated units are small molecules called monomers. Some of the molecules that serve as monomers have other f ...
... proteins, and nucleic acids—form chainlike molecules called polymers. A polymer is a long molecule consisting of many similar or identical building blocks linked by covalent bonds. The repeated units are small molecules called monomers. Some of the molecules that serve as monomers have other f ...
proteins aminacids notesKelly
... • changes in pH (alters electrostatic interactions between charged amino acids) • changes in salt concentration (does the same) • changes in temperature (higher temperatures reduce the strength of hydrogen bonds) • presence of reducing agents (break S-S bonds between cysteines) ...
... • changes in pH (alters electrostatic interactions between charged amino acids) • changes in salt concentration (does the same) • changes in temperature (higher temperatures reduce the strength of hydrogen bonds) • presence of reducing agents (break S-S bonds between cysteines) ...
biochemistry project
... Page 8 Level 3 specialist (Bonus 10 points)- Find a newspaper/magazine article that discusses one of the above macromolecules. Print it and paste it on pg 8. Provide the title/date and author/source of the article. Write 3 questions a teacher might ask if they assigned that article to their class as ...
... Page 8 Level 3 specialist (Bonus 10 points)- Find a newspaper/magazine article that discusses one of the above macromolecules. Print it and paste it on pg 8. Provide the title/date and author/source of the article. Write 3 questions a teacher might ask if they assigned that article to their class as ...
2 - Warner Pacific College
... product of the Calvin-Benson cycle (aka the “dark-reactions” or “lightindependent reactions”) A) ATP B) glucose C) NADPH ...
... product of the Calvin-Benson cycle (aka the “dark-reactions” or “lightindependent reactions”) A) ATP B) glucose C) NADPH ...
Analysis of energy metabolism in acetic acid bacteria during
... In both A. aceti and A. pasteurianus, the genes for the tricarboxylic acid (TCA) cycle enzymes were found to be significantly repressed when ethanol was present in the medium, even in the presence of glucose or acetate.1,2) Acetobacter species are able to produce proton motive force that is used for ...
... In both A. aceti and A. pasteurianus, the genes for the tricarboxylic acid (TCA) cycle enzymes were found to be significantly repressed when ethanol was present in the medium, even in the presence of glucose or acetate.1,2) Acetobacter species are able to produce proton motive force that is used for ...
Class22 2-9 Win17 Respiration Regulation and
... transformed into the ‘sticky’ 2-carbon Acetyl-CoA – Krebs Cycle: Acetyl-CoA feeds the Krebs cycle, which uses the oxidation of carbohydrates to form reducing power (as NADH, FADH2) – Electron Transport Chain: High-energy electrons are driven through membrane proteins that pump protons to produce a ...
... transformed into the ‘sticky’ 2-carbon Acetyl-CoA – Krebs Cycle: Acetyl-CoA feeds the Krebs cycle, which uses the oxidation of carbohydrates to form reducing power (as NADH, FADH2) – Electron Transport Chain: High-energy electrons are driven through membrane proteins that pump protons to produce a ...
1D17 – BMI201 Page 1 of 3 Code Questions Answers 1 Discuss the
... Enzymes are highly specific in their reaction. Occurrence of thousands of enzymes in biological system might be due to this specific nature of enzymes. There are three types of enzyme specificity and they are as follows: 1. Stereospecificity: also called optical specificity. Stereo isomer are the su ...
... Enzymes are highly specific in their reaction. Occurrence of thousands of enzymes in biological system might be due to this specific nature of enzymes. There are three types of enzyme specificity and they are as follows: 1. Stereospecificity: also called optical specificity. Stereo isomer are the su ...
Student notes part 6
... Heterotrophic Metabolism • Heterotrophic is what people are: – We get our energy from organic molecules taken in from our surroundings – food. Although heterotrophs may feed partially or exclusively on other heterotrophs, all the food molecules come ultimately from autotrophs. – Ability to brea ...
... Heterotrophic Metabolism • Heterotrophic is what people are: – We get our energy from organic molecules taken in from our surroundings – food. Although heterotrophs may feed partially or exclusively on other heterotrophs, all the food molecules come ultimately from autotrophs. – Ability to brea ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.