intermediary metabolism
... the cell for maintenance or growth are interrelated and well coordinated. This interrelation of metabolism could be considered at the following levels: A. The flow of key metabolites between different metabolic pathways at the cellular level. B. The interdependence of different organs and tissues in ...
... the cell for maintenance or growth are interrelated and well coordinated. This interrelation of metabolism could be considered at the following levels: A. The flow of key metabolites between different metabolic pathways at the cellular level. B. The interdependence of different organs and tissues in ...
Product Data Sheet - Max Muscle Sports Nutrition
... • Powerful Antioxidant to Reduce Oxidative Stress† ...
... • Powerful Antioxidant to Reduce Oxidative Stress† ...
Lipids
... phosphate (DHAP), a glycolytic intermediate. The free F.As produced are either reesterified to TAG in the adipose tissue or travel in the blood to be taken up by the cells for oxidation. ...
... phosphate (DHAP), a glycolytic intermediate. The free F.As produced are either reesterified to TAG in the adipose tissue or travel in the blood to be taken up by the cells for oxidation. ...
Comparative physiological studies on lour species of
... ly than the ions ( 1 0) . This account for the differences observed at the twu di fferent pH's. It may also be postulated that the lower pH wiU modify the per meability properties of the ceU membrane making the substrate available . to the enzymes. Furthermore, the data suggest that the tricarboxyl ...
... ly than the ions ( 1 0) . This account for the differences observed at the twu di fferent pH's. It may also be postulated that the lower pH wiU modify the per meability properties of the ceU membrane making the substrate available . to the enzymes. Furthermore, the data suggest that the tricarboxyl ...
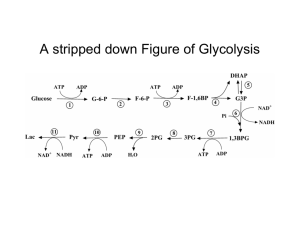
Figure 17-3 Degradation of glucose via the glycolytic pathway.
... •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skeletal muscle during periods of strenuous exertion: Cells use O2 faster than can be supplied by circulatory system; ce ...
... •lactate, end-product under anaerobic conditions, diffuses thru cell membrane as waste into blood - salvaged by liver and rebuilt to form glucose (gluconeogenesis). This occurs in skeletal muscle during periods of strenuous exertion: Cells use O2 faster than can be supplied by circulatory system; ce ...
Citric Acid Cycle Regulation
... If no Oxygen around, cant enter Citric Acid Cycle (CAC). Can only do glycolysis. Each round of glycolysis produces a net gain of 2 ATPs. Better than nothing so use glycolysis. But supply of NAD+ is limited in cytoplasm so must regenerate it to allow glycolysis to continue! Step 5 of glycolysis conve ...
... If no Oxygen around, cant enter Citric Acid Cycle (CAC). Can only do glycolysis. Each round of glycolysis produces a net gain of 2 ATPs. Better than nothing so use glycolysis. But supply of NAD+ is limited in cytoplasm so must regenerate it to allow glycolysis to continue! Step 5 of glycolysis conve ...
Fat - Food a fact of life
... Protein Protein is needed for growth and repair of the body. Excess protein can be broken down and used as a source of energy. Protein is made up of different combinations of amino acids. These are the building blocks of protein. Amino acids are compounds containing carbon, hydrogen, oxygen, nitrog ...
... Protein Protein is needed for growth and repair of the body. Excess protein can be broken down and used as a source of energy. Protein is made up of different combinations of amino acids. These are the building blocks of protein. Amino acids are compounds containing carbon, hydrogen, oxygen, nitrog ...
Attomole Detection of Proteins in a Complex Mixture Using the
... A standard protein mixture containing equal molar amounts of yeast enolase, bovine serum albumin, rabbit glycogen phosphorylase B, and yeast alcohol dehydrogenase, were spiked at various concentrations into 100 ng of digested E.coli cell lysate. This provided a dilution series ranging from 100 amol ...
... A standard protein mixture containing equal molar amounts of yeast enolase, bovine serum albumin, rabbit glycogen phosphorylase B, and yeast alcohol dehydrogenase, were spiked at various concentrations into 100 ng of digested E.coli cell lysate. This provided a dilution series ranging from 100 amol ...
7th International Symposium on
... M.J. Renne, B. Rasmussen, M. Sheffield-Moore, S. Schiaffino, P. Tessari, G. Toffolo, E. Volpi, F.M. White. The sessions and topics discussed in the Symposium are listed below: 1st session: Protein wasting in human disease: pathophysiological and clinical aspects Molecular and cellular mechanisms o ...
... M.J. Renne, B. Rasmussen, M. Sheffield-Moore, S. Schiaffino, P. Tessari, G. Toffolo, E. Volpi, F.M. White. The sessions and topics discussed in the Symposium are listed below: 1st session: Protein wasting in human disease: pathophysiological and clinical aspects Molecular and cellular mechanisms o ...
Sample Exam 2
... 45. Pinocytosis literally translates to “cell eating”. 46. Carriers change shape when moving substances across the cell membrane. 47. Osmosis is a form of passive transport. 48. Either (both) of the strands in a molecule of DNA can be used for the process of protein synthesis. 49. TAG and GTC are ex ...
... 45. Pinocytosis literally translates to “cell eating”. 46. Carriers change shape when moving substances across the cell membrane. 47. Osmosis is a form of passive transport. 48. Either (both) of the strands in a molecule of DNA can be used for the process of protein synthesis. 49. TAG and GTC are ex ...
Citric acid cycle ELECTRON TRANSPORT CHAIN AND
... 6.6 Redox reactions release energy when electrons “fall” from a hydrogen carrier to oxygen • NADH delivers electrons to a series of electron carriers in an electron transport chain – As electrons move from carrier to carrier, their energy is released in small quantities ...
... 6.6 Redox reactions release energy when electrons “fall” from a hydrogen carrier to oxygen • NADH delivers electrons to a series of electron carriers in an electron transport chain – As electrons move from carrier to carrier, their energy is released in small quantities ...
C6H12O6 + 6 O2* 6 CO2 + 6H2O + 38 ATP
... 3. ELECTRON TRANSPORT CHAIN In inner membrane of mitochondrion Largest energy making step “ATP Converter”- converts NADH + FADH2 into ATP Movement of Hydrogens (protons) fuels the process 3 Proteins (electron acceptors), each one is more electronegative than the first. By-product: H2O ...
... 3. ELECTRON TRANSPORT CHAIN In inner membrane of mitochondrion Largest energy making step “ATP Converter”- converts NADH + FADH2 into ATP Movement of Hydrogens (protons) fuels the process 3 Proteins (electron acceptors), each one is more electronegative than the first. By-product: H2O ...
Metabolism - Catabolism of Proteins & Fats Lecture PowerPoint
... • Images used on this resource, and on the SPO website are, wherever possible, credited and linked to their source. Any words underlined and appearing in blue are links that can be clicked on for more information. PowerPoints must be viewed in slide show mode to use the hyperlinks directly. • Severa ...
... • Images used on this resource, and on the SPO website are, wherever possible, credited and linked to their source. Any words underlined and appearing in blue are links that can be clicked on for more information. PowerPoints must be viewed in slide show mode to use the hyperlinks directly. • Severa ...
AP Biology
... – Proteins are polymers of amino acids joined by peptide bonds – All amino acids have a similar structure – All contain amino and carboxyl groups – All have a variable “R” group – Some R groups are hydrophobic – Some are hydrophilic ...
... – Proteins are polymers of amino acids joined by peptide bonds – All amino acids have a similar structure – All contain amino and carboxyl groups – All have a variable “R” group – Some R groups are hydrophobic – Some are hydrophilic ...
Molecular Biology of the Cell
... During power exercises in which the rate of demand for energy is high, lactate is produced faster than the ability of the tissues to remove it, so lactate concentration begins to rise. Contrary to popular belief, this increased concentration of lactate does not directly cause acidosis, nor is it res ...
... During power exercises in which the rate of demand for energy is high, lactate is produced faster than the ability of the tissues to remove it, so lactate concentration begins to rise. Contrary to popular belief, this increased concentration of lactate does not directly cause acidosis, nor is it res ...
CHAPTER 5 The Structure and Function of Macromolecules The
... a bilayer. The polar “head” is positioned toward the outside and inside of the cell, which has an affinity for the aqueous environment found both outside and inside the cell. The fatty acid tails of each layer of phospholipids are positioned toward the center of the membrane due to their nonpolar (w ...
... a bilayer. The polar “head” is positioned toward the outside and inside of the cell, which has an affinity for the aqueous environment found both outside and inside the cell. The fatty acid tails of each layer of phospholipids are positioned toward the center of the membrane due to their nonpolar (w ...
6-1
... Cells will use the energy in carbohydrates first. – Complex carbohydrates are metabolized into simple sugars. Cells can use the energy in fats and proteins as well. – Fats are digested into fatty acids and glycerol. – Proteins are digested into amino acids. Cells must convert fats and proteins into ...
... Cells will use the energy in carbohydrates first. – Complex carbohydrates are metabolized into simple sugars. Cells can use the energy in fats and proteins as well. – Fats are digested into fatty acids and glycerol. – Proteins are digested into amino acids. Cells must convert fats and proteins into ...
HOW CELLS HARVEST ENERGY
... carbs proteins fats Breakdown of these large organic molecules releases ATP which is used for work (primarily to drive endergonic reactions) Energy stored in chemical bonds is potential energy Energy released when those bonds are broken is kinetic energy I. ...
... carbs proteins fats Breakdown of these large organic molecules releases ATP which is used for work (primarily to drive endergonic reactions) Energy stored in chemical bonds is potential energy Energy released when those bonds are broken is kinetic energy I. ...
Sources of enzyme
... catalysis by a separated series of the enzymes. such enzyme preparations are kinetically more simple than the integrated living organisms from which they are produced ...
... catalysis by a separated series of the enzymes. such enzyme preparations are kinetically more simple than the integrated living organisms from which they are produced ...
Seed Germination and Reserve Mobilization
... the storage proteins, rendering them soluble, and susceptible to further proteolysis. Endopeptidases of a ‘proteinase B’ class and carboxypeptidases are then able to hydrolyse the modified storage proteins to produce small oligopeptides and amino acids. These reactions occur within the protein bodies ...
... the storage proteins, rendering them soluble, and susceptible to further proteolysis. Endopeptidases of a ‘proteinase B’ class and carboxypeptidases are then able to hydrolyse the modified storage proteins to produce small oligopeptides and amino acids. These reactions occur within the protein bodies ...
Document
... routine “Protein Assays” using colorimetric methods, it is still an approximation and amino acid absorption can be considerably altered by the local environment in the protein. There is a web site ProtParam, http://ca.expasy.org/tools/protparam.htmlthat can be used to estimate protein extinction coe ...
... routine “Protein Assays” using colorimetric methods, it is still an approximation and amino acid absorption can be considerably altered by the local environment in the protein. There is a web site ProtParam, http://ca.expasy.org/tools/protparam.htmlthat can be used to estimate protein extinction coe ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.