How does a Bohm particle localize?
... arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., should also be most interesting, particularly their variation with magnetic flux. In a fully locali ...
... arises without internal contradictions as the Bohm trajectories are not allowed to cross each other. The comparison of the trajectories to the semi-classical characteristics such as scar states, etc., should also be most interesting, particularly their variation with magnetic flux. In a fully locali ...
Quantum Potpourri
... Electrons in atoms or molecules are characterized by their entire distributions, called wave functions or orbitals, rather than by instantaneous positions and velocities: an electron may be considered always to be, with appropriate probability, at all points of its distribution, which does not vary ...
... Electrons in atoms or molecules are characterized by their entire distributions, called wave functions or orbitals, rather than by instantaneous positions and velocities: an electron may be considered always to be, with appropriate probability, at all points of its distribution, which does not vary ...
Recap of Lectures 9-11
... Principle of Superposition: quantum states show interference and require both an amplitude and a phase for the parts Superposition applies in time as well as space For any observable, measured values come from a particular set of possibilities (sometimes quantised). Some states (eigenstates) always ...
... Principle of Superposition: quantum states show interference and require both an amplitude and a phase for the parts Superposition applies in time as well as space For any observable, measured values come from a particular set of possibilities (sometimes quantised). Some states (eigenstates) always ...
Quantum Mechanics
... paradox of the CI formulated by Einstein, Boris Podolsky, and Nathan Rosen in reaction to the CI quantum entanglement – connection of two or more particles anti-correlation of e- and e+ spin if spin is measured in one, the wavefunction of the other collapses; superluminal information transfer Copenh ...
... paradox of the CI formulated by Einstein, Boris Podolsky, and Nathan Rosen in reaction to the CI quantum entanglement – connection of two or more particles anti-correlation of e- and e+ spin if spin is measured in one, the wavefunction of the other collapses; superluminal information transfer Copenh ...
Quantum Measurements PHYSICS COLLOQUIUM Klaus Mølmer
... Abstract: The famous discussions between Niels Bohr and Albert Einstein on the interpretation of quantum mechanics did not resolve their main issue which concerned the indeterminacy of measurements on individual quantum systems, and even today there is no, commonly agreed upon, understanding of the ...
... Abstract: The famous discussions between Niels Bohr and Albert Einstein on the interpretation of quantum mechanics did not resolve their main issue which concerned the indeterminacy of measurements on individual quantum systems, and even today there is no, commonly agreed upon, understanding of the ...
SCHRÖDINGER EQUATION FOR A PARTICLE ON A CURVED SPACE AND SUPERINTEGRABILITY
... of the linear momentum operator. Plane waves are therefore simultaneous eigenfunctions of energy and linear momentum. As soon as the problem is thought of in a space with curvature, the analysis becomes much more complicated [11, 14, 15]. First of all, the canonical momenta do not in general coincid ...
... of the linear momentum operator. Plane waves are therefore simultaneous eigenfunctions of energy and linear momentum. As soon as the problem is thought of in a space with curvature, the analysis becomes much more complicated [11, 14, 15]. First of all, the canonical momenta do not in general coincid ...
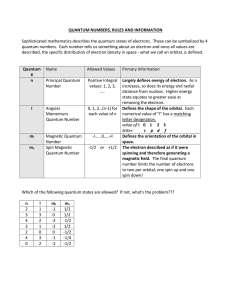
Quantum Number Table
... Sophisticated mathematics describes the quantum states of electrons. These can be symbolized by 4 quantum numbers. Each number tells us something about an electron and once all values are described, the specific distribution of electron density in space - what we call an orbital, is defined. ...
... Sophisticated mathematics describes the quantum states of electrons. These can be symbolized by 4 quantum numbers. Each number tells us something about an electron and once all values are described, the specific distribution of electron density in space - what we call an orbital, is defined. ...
Does the Everyday World Really Obey Quantum Mechanics?
... Figure 1 Erwin Schrödinger (left) and Niels Bohr. Bohr claimed that a momentum kick, imparted by any measurement of particle position, could explain the disappearance of quantum interference in ‘two-slit’ experiments. A new experiment1 shows that this effect is too small, and the disappearance must ...
... Figure 1 Erwin Schrödinger (left) and Niels Bohr. Bohr claimed that a momentum kick, imparted by any measurement of particle position, could explain the disappearance of quantum interference in ‘two-slit’ experiments. A new experiment1 shows that this effect is too small, and the disappearance must ...
WAVE MECHANICS AND QUANTUM NUMBERS
... 2. supported by the facts that electrons undergo diffraction and interference 3. Werner Heisenberg 1927- Heisenberg Uncertainty Principle: it is impossible to simultaneously identify the position and velocity of an electron, or any particle. 4. wave mechanics looks to suggest the locations of electr ...
... 2. supported by the facts that electrons undergo diffraction and interference 3. Werner Heisenberg 1927- Heisenberg Uncertainty Principle: it is impossible to simultaneously identify the position and velocity of an electron, or any particle. 4. wave mechanics looks to suggest the locations of electr ...