
Equilibrium Booklet - mrstorie
... The above graph shows concentration versus time for a system containing carbon monoxide (CO) dichlorine (Cl2) and phosgene (COCl2). ...
... The above graph shows concentration versus time for a system containing carbon monoxide (CO) dichlorine (Cl2) and phosgene (COCl2). ...
Lab announcements – 2 lab quiz week before spring break
... Lab announcements – 2nd lab quiz week before spring break photocopy before turning in or study before turning in -same as 101/other 102 sections be extra careful with dilutions this week (Expt. 21) Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur s ...
... Lab announcements – 2nd lab quiz week before spring break photocopy before turning in or study before turning in -same as 101/other 102 sections be extra careful with dilutions this week (Expt. 21) Most chemical reactions do not go to completion. chemical equilibrium – two opposing reactions occur s ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... that the Mo is more difficult to reduce after being placed onto the AC support, and the addition of a K promoter greatly promotes the formation of Mo species reducible at relatively low temperatures, while it retards the generation of Mo species that are reducible only at higher temperatures. These ...
... that the Mo is more difficult to reduce after being placed onto the AC support, and the addition of a K promoter greatly promotes the formation of Mo species reducible at relatively low temperatures, while it retards the generation of Mo species that are reducible only at higher temperatures. These ...
Solubility and Solubility Equilibrium
... about equilibrium for insoluble species, but we can talk about equilibrium of insoluble molecules because, to some small extent, all insoluble products dissolve in solution and reach an equilibrium. Everything dissolves in everything and there's a little bit of everything, everywhere. A little bit c ...
... about equilibrium for insoluble species, but we can talk about equilibrium of insoluble molecules because, to some small extent, all insoluble products dissolve in solution and reach an equilibrium. Everything dissolves in everything and there's a little bit of everything, everywhere. A little bit c ...
2008 Equilibrium -- without math (PowerPoint 13 MB)
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
... between forward and reverse reactions. In most cases, this balance is quite delicate. Changes in experimental conditions (concentration, pressure, volume and temperature) may disturb the balance and shift the equilibrium position so that more or less of the desired product is formed. When we say tha ...
Ch16 - WordPress.com
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
spontaneous change: entropy and free energy
... not depend on the gas pressure, but only on the temperature. Therefore, in this expansion ¢U = 0. Also, the enthalpy change is zero: ¢H = 0. This means that the expansion is not caused by the system dropping to a lower energy state. A convenient mental image to “explain” the expansion is that the ga ...
... not depend on the gas pressure, but only on the temperature. Therefore, in this expansion ¢U = 0. Also, the enthalpy change is zero: ¢H = 0. This means that the expansion is not caused by the system dropping to a lower energy state. A convenient mental image to “explain” the expansion is that the ga ...
Chapter 3 PowerPoint Presentation
... • Every substance has its own unique set of physical and chemical properties. • Observations of properties may vary depending on the conditions of the immediate environment. • It is important to state the specific conditions in which observations are made because both chemical and physical propertie ...
... • Every substance has its own unique set of physical and chemical properties. • Observations of properties may vary depending on the conditions of the immediate environment. • It is important to state the specific conditions in which observations are made because both chemical and physical propertie ...
Ch16
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
15.0 EquilibriumIHS2014
... Catalysts speed up the rate at which equilibrium is obtained, but have no effect on the magnitude of K. They increase both the forward and backward rate of reaction. ...
... Catalysts speed up the rate at which equilibrium is obtained, but have no effect on the magnitude of K. They increase both the forward and backward rate of reaction. ...
MOF-74 building unit has a direct impact on toxic gas
... breakthrough tests were run within the same month of synthesis of the MOF material. For the Zn analog, the testing was spread out over a period of 8 months after materials synthesis. For the Mg analog, most breakthrough testing was completed within a month of the synthesis of the material. However, ...
... breakthrough tests were run within the same month of synthesis of the MOF material. For the Zn analog, the testing was spread out over a period of 8 months after materials synthesis. For the Mg analog, most breakthrough testing was completed within a month of the synthesis of the material. However, ...
Chapter 16 Controlling the yield of reactions
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
... The entries in the first column indicate the initial amount that was in the closed vessel, the amount that was used that is calculated from stoichiometry and the amount that remains (or is in excess) and is present at equilibrium, calculated by subtraction (initial – used). Equilibrium constants are ...
PURPOSE: To determine the value of the equilibrium constant for a
... There is a universal tendency of metal ions to form complexes with negatively charged ions (e.g. SCN -) and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between ...
... There is a universal tendency of metal ions to form complexes with negatively charged ions (e.g. SCN -) and with neutral molecules (e.g., water, H2O, and ammonia, NH3) that have lone pairs of electrons (nonbonding pairs). This tendency can be understood as the result of the attractive force between ...
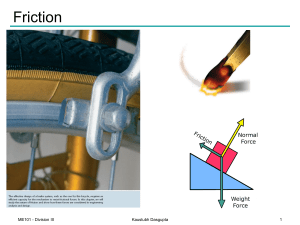
Friction
... • Maximum static-friction force and kineticfriction force are: - proportional to normal force - dependent on type and condition of contact surfaces A friction coefficient reflects roughness, which is a geometric property of surfaces - independent of contact area Surfaces in relative motion :: the co ...
... • Maximum static-friction force and kineticfriction force are: - proportional to normal force - dependent on type and condition of contact surfaces A friction coefficient reflects roughness, which is a geometric property of surfaces - independent of contact area Surfaces in relative motion :: the co ...
Use the following answers for questions 1
... 39. Equal masses of three different ideal Gas, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It is equal to 1/3 the total pressure (B) It depends on the inte ...
... 39. Equal masses of three different ideal Gas, X, Y, and Z, are mixed in a sealed rigid container. If the temperature of the system remains constant, which of the following statements about the partial pressure of gas X is correct? (A) It is equal to 1/3 the total pressure (B) It depends on the inte ...
Chapter 14: Chemical Equilibrium
... to the guidelines stated above for effect of changing concentration. ...
... to the guidelines stated above for effect of changing concentration. ...
Physical Chemistry 1.pdf
... often place more emphasis on speeding up the rate of a reaction than on its percentage yield. Organic chemists use kinetic studies to determine the mechanisms of reactions and to tell how fast products will be formed. ...
... often place more emphasis on speeding up the rate of a reaction than on its percentage yield. Organic chemists use kinetic studies to determine the mechanisms of reactions and to tell how fast products will be formed. ...
fast pyrolysis characteristics of sugarcane bagasse hemicellulose
... Fast pyrolysis of hemicellulose and product distribution The distributions of products resulted from the pyrolysis of sugarcane bagasse hemicellulose at different temperatures in the tubular furnace are shown in Figure 2. As seen, the product distributions of hemicellulose changed with increasing te ...
... Fast pyrolysis of hemicellulose and product distribution The distributions of products resulted from the pyrolysis of sugarcane bagasse hemicellulose at different temperatures in the tubular furnace are shown in Figure 2. As seen, the product distributions of hemicellulose changed with increasing te ...
4.6 M - Thierry Karsenti
... situation in which the reactants and products are producing each other at the same rate. Exothermic process. A process that releases energy as heat. Free energy (G). The energy available to do useful work. Defined by G = H − TΔS. Changes in free energy are useful in indicating the conditions under w ...
... situation in which the reactants and products are producing each other at the same rate. Exothermic process. A process that releases energy as heat. Free energy (G). The energy available to do useful work. Defined by G = H − TΔS. Changes in free energy are useful in indicating the conditions under w ...
Test bank questions
... 50.0 g of N2O4 is introduced into an evacuated 2.00 L vessel and allowed to come to equilibrium with its decomposition product, N2O4(g) 2NO2(g). For this reaction Kc = 0.133. Once the system has reached equilibrium, 5.00 g of NO 2 is injected into the vessel, and the system is allowed to equilibrate ...
... 50.0 g of N2O4 is introduced into an evacuated 2.00 L vessel and allowed to come to equilibrium with its decomposition product, N2O4(g) 2NO2(g). For this reaction Kc = 0.133. Once the system has reached equilibrium, 5.00 g of NO 2 is injected into the vessel, and the system is allowed to equilibrate ...
13 CHEMICAL EQUILIBRIUM W MODULE - 5
... and reverse reactions become equal; and all the properties of the system become constant. It is said that the system has attained state of equilibration. However it may be noted that the state of equilibrium is reached only if the reaction is carried out in a closed system. At the time of equilibriu ...
... and reverse reactions become equal; and all the properties of the system become constant. It is said that the system has attained state of equilibration. However it may be noted that the state of equilibrium is reached only if the reaction is carried out in a closed system. At the time of equilibriu ...
Unit 7 Reaction Rates and Equilibrium Notes
... Reversible Reactions: - reactions that can go from the right hand side of the equation (products) to the left hand side of the equation (reactants). Chemical Equilibrium: - the state at which the concentrations of all reactants and products remain constant with time (the Forward Reaction Rate = Reve ...
... Reversible Reactions: - reactions that can go from the right hand side of the equation (products) to the left hand side of the equation (reactants). Chemical Equilibrium: - the state at which the concentrations of all reactants and products remain constant with time (the Forward Reaction Rate = Reve ...
Keq = [A] [B] [C] [D]
... b) Substitute the concentration terms with the equilibrium values from the ICE table, and the value of Kc: 6.0 x 10-9 = x · x c) Solve for x by taking the square root of both sides: 6.0 x 10-9 = x2 and x = 7.7 x 10-5 mol/L d) Therefore, the concentration of both NH3 and HCl gases at equilibrium wil ...
... b) Substitute the concentration terms with the equilibrium values from the ICE table, and the value of Kc: 6.0 x 10-9 = x · x c) Solve for x by taking the square root of both sides: 6.0 x 10-9 = x2 and x = 7.7 x 10-5 mol/L d) Therefore, the concentration of both NH3 and HCl gases at equilibrium wil ...
Nutrient uptake by protocells: a liposome model system
... (Petkau and Chelack, 1972; Stillwell and Winter, 1974). In these studies, the increase of ATP permeability was found to be related to the presence of divalent cations in the solutions. However, these bilayers contained lipids with charged headgroups that could have directly interacted with metal ion ...
... (Petkau and Chelack, 1972; Stillwell and Winter, 1974). In these studies, the increase of ATP permeability was found to be related to the presence of divalent cations in the solutions. However, these bilayers contained lipids with charged headgroups that could have directly interacted with metal ion ...




















![Keq = [A] [B] [C] [D]](http://s1.studyres.com/store/data/014463360_1-50a2de0db1e8b9a361c4b31c6e85c28d-300x300.png)
