Equilibrium STUDY GUIDE by Keshara Senanayake ---
... Q is less than Keq (Q < Keq), the denominator of the reaction quotient expression is too large and the numerator is too small. This means that at the time of measurement there is too much of the reactants and too little of the products. The reaction will consume reactants and form the products to re ...
... Q is less than Keq (Q < Keq), the denominator of the reaction quotient expression is too large and the numerator is too small. This means that at the time of measurement there is too much of the reactants and too little of the products. The reaction will consume reactants and form the products to re ...
SAMPLE AP CHEMISTRY EXAM QUESTIONS
... An unknown compound contains only the three elements C, H, and O. A pure sample of the compound is analyzed and found to be 65.60 percent C and 9.44 percent H by mass. (a) Determine the empirical formula of the compound. (b) A solution of 1.570 grams of the compound in 16.08 grams of camphor is obse ...
... An unknown compound contains only the three elements C, H, and O. A pure sample of the compound is analyzed and found to be 65.60 percent C and 9.44 percent H by mass. (a) Determine the empirical formula of the compound. (b) A solution of 1.570 grams of the compound in 16.08 grams of camphor is obse ...
Deuterium fractionation of methylamine through atomic grain
... amino acid in space [2]. Laboratory studies revealed that methylamine can be formed by various reactions both in the gas phase and the solid phase in molecular clouds (MCs) [3,4]. In either case, it is likely that the CH3NH2 is retained on icy grains at as low as 10 K and is subjected to various pro ...
... amino acid in space [2]. Laboratory studies revealed that methylamine can be formed by various reactions both in the gas phase and the solid phase in molecular clouds (MCs) [3,4]. In either case, it is likely that the CH3NH2 is retained on icy grains at as low as 10 K and is subjected to various pro ...
Stoichiometry, Lab Basics, Reactions
... adding Na2CO3 (s) to 2.5 M HCl. The student sets up the apparatus to collect the CO2 gas over water. The volume of gas collected is much less than the expected volume because CO2 gas: A) is very soluble in water. B) is produced at a low pressure. C) is more dense than water vapor. D) has a larger mo ...
... adding Na2CO3 (s) to 2.5 M HCl. The student sets up the apparatus to collect the CO2 gas over water. The volume of gas collected is much less than the expected volume because CO2 gas: A) is very soluble in water. B) is produced at a low pressure. C) is more dense than water vapor. D) has a larger mo ...
Chemistry
... DO NOT write anything else on the Test Booklet. 4. This Booklet contains 120 items (questions). Each item comprises four responses (answers). You will select one response which you want to mark on the Response Sheet. In case you feel that there is more than one correct response, mark the response wh ...
... DO NOT write anything else on the Test Booklet. 4. This Booklet contains 120 items (questions). Each item comprises four responses (answers). You will select one response which you want to mark on the Response Sheet. In case you feel that there is more than one correct response, mark the response wh ...
The First Law of Thermodynamics
... the area under the curve (Figure 3.2b); because the external pressure is no longer held constant, however, the area is considerably greater. From the foregoing discussion, we can draw several conclusions about work. First, work should be thought of as a mode of energy transfer. Gas expands because t ...
... the area under the curve (Figure 3.2b); because the external pressure is no longer held constant, however, the area is considerably greater. From the foregoing discussion, we can draw several conclusions about work. First, work should be thought of as a mode of energy transfer. Gas expands because t ...
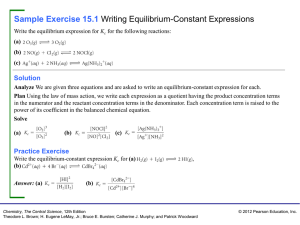
Sample Exercise 15.1 Writing Equilibrium
... Theodore L. Brown; H. Eugene LeMay, Jr.; Bruce E. Bursten; Catherine J. Murphy; and Patrick Woodward ...
... Theodore L. Brown; H. Eugene LeMay, Jr.; Bruce E. Bursten; Catherine J. Murphy; and Patrick Woodward ...
Follow Along Notes - Jackson County School System
... CO2(g) + H2(g) H2O(g) + CO(g) When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction ...
... CO2(g) + H2(g) H2O(g) + CO(g) When H2(g) is mixed with CO2(g) at 2,000 K, equilibrium is achieved according to the equation above. In one experiment, the following equilibrium concentrations were measured. [H2] = 0.20 mol/L [CO2] = 0.30 mol/L [H2O] = [CO] = 0.55 mol/L (a) What is the mole fraction ...
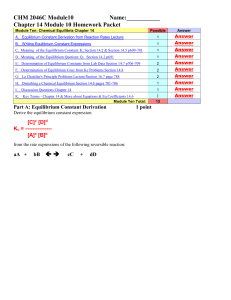
CHEM 1212 Module Ten-Chapter 16 Name
... contributes to air pollution whenever fuel is burned at high temperatures and high pressure in an automobile gasoline engine. At 1500 K, K= 1.0 x 10 -5. Suppose a sample of air has [N2] = 0.80 M and [O2] = 0.20 M before any reaction occurs. Calculate the equilibrium concentrations of the reactants a ...
... contributes to air pollution whenever fuel is burned at high temperatures and high pressure in an automobile gasoline engine. At 1500 K, K= 1.0 x 10 -5. Suppose a sample of air has [N2] = 0.80 M and [O2] = 0.20 M before any reaction occurs. Calculate the equilibrium concentrations of the reactants a ...
Chapter 1 Introduction: Matter and Measurement
... For a Fe metal object whose density is 7.86 g/mL. (a) What is the mass (g) of a piece of this metal if it displaces 12. mL of water in a graduated cylinder? ...
... For a Fe metal object whose density is 7.86 g/mL. (a) What is the mass (g) of a piece of this metal if it displaces 12. mL of water in a graduated cylinder? ...
File
... Sodium thiosulfate solution, Na2S2O3(aq), and hydrochloric acid, HCl(aq), react spontaneously to produce solid sulfur, S(s), according to the equation below. S2O32–(aq) + 2H+(aq) → S(s) + SO2(aq) + H2O(l) A student experimentally determined the rate expression to be: rate = k[S2O32–(aq)]2 Which grap ...
... Sodium thiosulfate solution, Na2S2O3(aq), and hydrochloric acid, HCl(aq), react spontaneously to produce solid sulfur, S(s), according to the equation below. S2O32–(aq) + 2H+(aq) → S(s) + SO2(aq) + H2O(l) A student experimentally determined the rate expression to be: rate = k[S2O32–(aq)]2 Which grap ...
Non-ideal Plastic Behavior
... properties. Unfortunately, the properties of the raw ingredients and the processing conditions vary from time to time which causes the properties of the final product to vary, often in an unpredictable way. How can food manufacturers control these variations? Firstly, they can understand the role th ...
... properties. Unfortunately, the properties of the raw ingredients and the processing conditions vary from time to time which causes the properties of the final product to vary, often in an unpredictable way. How can food manufacturers control these variations? Firstly, they can understand the role th ...
Reaction Rates/Chemical Kinetics
... In this reaction, SO2 and O2 are placed in a container. Initially, the forward reaction proceeds and SO3 is produced. The rate of the forward reaction is much greater than the rate of the reverse reaction. As SO3 builds up, it starts to decompose into SO2 and O2. The rate of the forward reaction is ...
... In this reaction, SO2 and O2 are placed in a container. Initially, the forward reaction proceeds and SO3 is produced. The rate of the forward reaction is much greater than the rate of the reverse reaction. As SO3 builds up, it starts to decompose into SO2 and O2. The rate of the forward reaction is ...
Chemistry 30 - SharpSchool
... experiments have shown that under a given set of conditions (P and T) a specific quantitative relationship exists between the equilibrium concentrations of the reactants and products one reaction that has been studied intensively is that between H2(g) and I2(g) (simple molecules and takes place in g ...
... experiments have shown that under a given set of conditions (P and T) a specific quantitative relationship exists between the equilibrium concentrations of the reactants and products one reaction that has been studied intensively is that between H2(g) and I2(g) (simple molecules and takes place in g ...
Chemical Equilibrium
... 1. How many moles per liter of silver chloride will be in a saturated solution of AgCl? Ksp = 1.8x10-10 AgCl(s) Ag+(aq) + Cl-(aq) ...
... 1. How many moles per liter of silver chloride will be in a saturated solution of AgCl? Ksp = 1.8x10-10 AgCl(s) Ag+(aq) + Cl-(aq) ...
Chap15 - Bakersfield College
... reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The reaction quotient, Qc, is an expression that has the same form as the equilibrium-constant expression but whose concentrations are not necessari ...
... reestablish equilibrium? – To answer this question, substitute the current concentrations into the reaction quotient expression and compare it to Kc. – The reaction quotient, Qc, is an expression that has the same form as the equilibrium-constant expression but whose concentrations are not necessari ...
2011-2012 ACAD REVIEW SHEET Chapter 16
... How is the reaction quotient related to the equilibrium constant? In other words, how are they different, the same? (ANS: They differ in the time they are measured; K is measured at equilibrium only and Q is measured at anytime. They are the same with respect to their equation; they have the same eq ...
... How is the reaction quotient related to the equilibrium constant? In other words, how are they different, the same? (ANS: They differ in the time they are measured; K is measured at equilibrium only and Q is measured at anytime. They are the same with respect to their equation; they have the same eq ...
Ch. 18 Class PowerPoint
... saturated solution with a specific amount of solvent. • It has an infinite number of possible values at a given temperature and is dependent on other conditions, such as the presence of a common ion. ...
... saturated solution with a specific amount of solvent. • It has an infinite number of possible values at a given temperature and is dependent on other conditions, such as the presence of a common ion. ...
Document
... Ammonia is prepared industrially by the reaction: N2(g) + 3H2(g) 2NH3(g) For the reaction, H° = – 92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following is true? A) K for the reaction will be larger at 500°C than at 25°C. B) At equili ...
... Ammonia is prepared industrially by the reaction: N2(g) + 3H2(g) 2NH3(g) For the reaction, H° = – 92.2 kJ and K (at 25°C) = 4.0 × 108. When the temperature of the reaction is increased to 500°C, which of the following is true? A) K for the reaction will be larger at 500°C than at 25°C. B) At equili ...
Thermodynamics of Equilibrium
... Thus although for the four tosses there is a good chance (62%) that the H/T ratio will fall outside the range of 0.45 - 0.55, this probability becomes almost zero for 1000 tosses. To express this in a different way, the chances that 1000 gas molecules moving about randomly in a container would at an ...
... Thus although for the four tosses there is a good chance (62%) that the H/T ratio will fall outside the range of 0.45 - 0.55, this probability becomes almost zero for 1000 tosses. To express this in a different way, the chances that 1000 gas molecules moving about randomly in a container would at an ...
Measuring Rates
... The integrated rate law for a chemical reaction expresses how the concentration of a relevant reacting species changes as a function of time. Thus, it can be used to predict the time it will take for a reactant or product to reach a given concentration, or to predict such concentration at a selected ...
... The integrated rate law for a chemical reaction expresses how the concentration of a relevant reacting species changes as a function of time. Thus, it can be used to predict the time it will take for a reactant or product to reach a given concentration, or to predict such concentration at a selected ...
Properties of Systems in Equilibrium - Le
... salt solutions are mixed together resulting in the production of an insoluble salt. Notice that this process corresponds to a left shift of Reaction (11), and so Equation (12) can also be used to examine the conditions required for the precipitation of a solid to occur. We can denote the product [A+ ...
... salt solutions are mixed together resulting in the production of an insoluble salt. Notice that this process corresponds to a left shift of Reaction (11), and so Equation (12) can also be used to examine the conditions required for the precipitation of a solid to occur. We can denote the product [A+ ...
Engineering Thermodynamics
... The quantity denoted by Q in Equation 2.8 accounts for the amount of energy transferred to a closed system during a process by means other than work. On the basis of experiments it is known that such an energy transfer is induced only as a result of a temperature difference between the system and it ...
... The quantity denoted by Q in Equation 2.8 accounts for the amount of energy transferred to a closed system during a process by means other than work. On the basis of experiments it is known that such an energy transfer is induced only as a result of a temperature difference between the system and it ...
Chemical Thermodynamics presentation 1
... • Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. • Above 0 C it is spontaneous for ice to melt. • Below 0 C the reverse process is spontaneous. ...
... • Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. • Above 0 C it is spontaneous for ice to melt. • Below 0 C the reverse process is spontaneous. ...