Element - the simplest form of matter that can exist under normal
... Elements are the building blocks for all other substances There are now 117 known elements (as of 2006). All elements after uranium on the periodic table are man-made. A compound is a chemical combination of two or more different elements joined together in a fixed proportion. Every compound has its ...
... Elements are the building blocks for all other substances There are now 117 known elements (as of 2006). All elements after uranium on the periodic table are man-made. A compound is a chemical combination of two or more different elements joined together in a fixed proportion. Every compound has its ...
Chemical Reaction Basics
... Advanced Chemistry – Chapter 8 A ____________ ____________ is a process by which one or more substances are changed into one or more ____________ substances. Chemical reactions are represented by some type of ____________. The general form is as follows: ...
... Advanced Chemistry – Chapter 8 A ____________ ____________ is a process by which one or more substances are changed into one or more ____________ substances. Chemical reactions are represented by some type of ____________. The general form is as follows: ...
Ch 5.1 The Nature of Chemical Reactions
... Objectives For this Chapter • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
... Objectives For this Chapter • Understand parts to a chemical equation (reactants, products, yeild sign, double arrow) • Conservation of matter is expressed through balancing chemical equations • Describe difference between endothermic and exothermic reactions ...
Headline Text 28 Point Color Text 2
... Chemical informatics and medicinal chemistry databases How molecular motors work Computer modeling in support of chemical and drug design • Polymer delivery systems • Catalysts • Small molecule drugs ...
... Chemical informatics and medicinal chemistry databases How molecular motors work Computer modeling in support of chemical and drug design • Polymer delivery systems • Catalysts • Small molecule drugs ...
Chapter 2
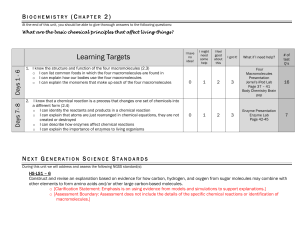
... Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. o [Clarification Statement: Emphasis is on using evidence from models and simulations to supp ...
... Construct and revise an explanation based on evidence for how carbon, hydrogen, and oxygen from sugar molecules may combine with other elements to form amino acids and/or other large carbon-based molecules. o [Clarification Statement: Emphasis is on using evidence from models and simulations to supp ...
Chemistry Content Standards
... physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. c. Analyzing (both conceptually and quantitatively) flow of energy during change of st ...
... physical processes. a. Compare and contrast atomic/molecular motion in solids, liquids, gases, and plasmas. b. Collect data and calculate the amount of heat given off or taken in by chemical or physical processes. c. Analyzing (both conceptually and quantitatively) flow of energy during change of st ...
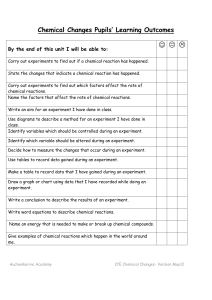
Chemical Reactions Unit Pupils` Learning Outcomes
... Chemical Changes Pupils’ Learning Outcomes ...
... Chemical Changes Pupils’ Learning Outcomes ...
Answer Key - La Quinta High School
... 1. All chemical reactions do produce some evidence that the reaction has occurred, but sometimes this evidence may not be visual and may not be very obvious. For example, when very dilute aqueous solutions of acids and bases are mixed, the neutralization reaction H+(aq) + OH–(aq) ! H2O(l) takes plac ...
... 1. All chemical reactions do produce some evidence that the reaction has occurred, but sometimes this evidence may not be visual and may not be very obvious. For example, when very dilute aqueous solutions of acids and bases are mixed, the neutralization reaction H+(aq) + OH–(aq) ! H2O(l) takes plac ...
Physical and Chemical Changes
... 4. What elements can organic compounds contain? List names, not symbols. Organic compounds MUST contain Carbon and Hydrogen; they may also contain Oxygen, Nitrogen, Sulfur, Phosphorus 5. What is the main function of the digestive system? The main function of the digestive system is to break down lar ...
... 4. What elements can organic compounds contain? List names, not symbols. Organic compounds MUST contain Carbon and Hydrogen; they may also contain Oxygen, Nitrogen, Sulfur, Phosphorus 5. What is the main function of the digestive system? The main function of the digestive system is to break down lar ...
Chemical Equations and Reactions notes File
... Reactants - original Products - resulting Reactants Products ...
... Reactants - original Products - resulting Reactants Products ...
Chemical Reactions
... Chapter Notes (pg.32-45) Chemical Change A __________________ _________________ occurs when the properties of the __________________, what you end up with, are different from the _________________, the starting substances. The following is an example of a chemical reaction: ...
... Chapter Notes (pg.32-45) Chemical Change A __________________ _________________ occurs when the properties of the __________________, what you end up with, are different from the _________________, the starting substances. The following is an example of a chemical reaction: ...
Deconstructed HS-PS1-2
... trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical rea ...
... trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical rea ...
Gizmos: Types of Reactions
... Introduction: Chemical equations show how compounds and elements react with one another. An element is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Questio ...
... Introduction: Chemical equations show how compounds and elements react with one another. An element is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Questio ...
Unit 2: Chemical Reactions
... • A chemical formula is an abbreviation for a chemical compound using chemical symbols and numbers. • The subscript number tells how many atoms of the element are present in the compound • Example: CO2 = Carbon Dioxide – Di = 2 – 1 Carbon atom and 2 oxygen atoms ...
... • A chemical formula is an abbreviation for a chemical compound using chemical symbols and numbers. • The subscript number tells how many atoms of the element are present in the compound • Example: CO2 = Carbon Dioxide – Di = 2 – 1 Carbon atom and 2 oxygen atoms ...
CHM 2045C - State College of Florida
... CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic conce ...
... CHM 1025C with a grade of “C” or better or one year of high school college preparatory honors or AP chemistry within last three years. This course meets Area V for the A.A./A.S. general education requirements. A rigorous study of chemistry principles for students who have already studied basic conce ...
Unit 2 Test Review
... Small molecules that link to form very large ones The monomer that makes up proteins A chemical that releases hydroxide (-OH) ions in a solution Any chemical that speeds up chemical reactions without being used up The chemical that an enzyme modifies Part of the enzyme that the substrate bonds to; s ...
... Small molecules that link to form very large ones The monomer that makes up proteins A chemical that releases hydroxide (-OH) ions in a solution Any chemical that speeds up chemical reactions without being used up The chemical that an enzyme modifies Part of the enzyme that the substrate bonds to; s ...
SNC2DExamChemistryreview
... Chemistry Exam Review 1. What is a chemical change? List how can you tell that a reaction or a material has had a chemical change? 2. In the earlier chemistry courses we learned about physical properties of materials. Give at least 5 different examples of physical properties? 3. Given the following ...
... Chemistry Exam Review 1. What is a chemical change? List how can you tell that a reaction or a material has had a chemical change? 2. In the earlier chemistry courses we learned about physical properties of materials. Give at least 5 different examples of physical properties? 3. Given the following ...
CHEMICAL INVENTORY - Web based software (chemoventory v3.0)
... SOFTWARE – Chemoventory 3.0 Chemoventory is a web ...
... SOFTWARE – Chemoventory 3.0 Chemoventory is a web ...
Unit 5 Chemical Properties and Changes Video Notes A ______ is a
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
Chemistry Unit
... 19. Write the equation for the neutralization reaction between hydrochloric acid and barium hydroxide. Then balance the reaction. 20. Chemical A has a pH of 3.2 and chemical B has a pH of 2.8. Which is more basic and WHY? 21. Chemical A has a pH of 8.2, chemical B has a pH of 12.5, chemical C has a ...
... 19. Write the equation for the neutralization reaction between hydrochloric acid and barium hydroxide. Then balance the reaction. 20. Chemical A has a pH of 3.2 and chemical B has a pH of 2.8. Which is more basic and WHY? 21. Chemical A has a pH of 8.2, chemical B has a pH of 12.5, chemical C has a ...
milli-extraction column for liquid-liquid phase separation
... LIQUID-LIQUID PHASE SEPARATION CHEMICAL PROCESS ENGINEERING Best Practice Example created by ...
... LIQUID-LIQUID PHASE SEPARATION CHEMICAL PROCESS ENGINEERING Best Practice Example created by ...
VX (nerve agent)

VX (IUPAC name O-ethyl S-[2-(diisopropylamino)ethyl] methylphosphonothioate) is an extremely toxic substance that has no known uses except in chemical warfare as a nerve agent. It is a tasteless and odorless liquid. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687. The production and stockpiling of VX exceeding 100 grams per year was outlawed by the Chemical Weapons Convention of 1993.The VX nerve agent is the best-known of the V-series of nerve agents and is considered an area denial weapon due to its physical properties.