Lecture 2: Atoms - U of L Class Index
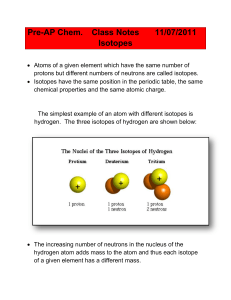
... number of protons in an atom (as in a nuclear reaction) changes the element. While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have more than one naturally occurring isotope: ...
... number of protons in an atom (as in a nuclear reaction) changes the element. While atoms of the same element must have the same atomic number, they may have different mass numbers. If so, they are referred to as isotopes. Most elements have more than one naturally occurring isotope: ...
Atomic Number, Mass Number, and Isotopes
... protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Although all atoms of an element have the same number of protons, they don’t all have t ...
... protons determines the identity of the atom. For example, a carbon atom always has six protons. If it has seven protons, it’s nitrogen, not carbon. The number of protons is called the atomic number (Z). ISOTOPES: Although all atoms of an element have the same number of protons, they don’t all have t ...
Exemplar exam question – Chapter 2
... The first answer is probably worthy of only 1 mark as it does not make clear that isotopes are different atoms of the same element. The second answer would probably score 0. Although the idea of the same element and different number of neutrons is mentioned, the student has not mentioned different a ...
... The first answer is probably worthy of only 1 mark as it does not make clear that isotopes are different atoms of the same element. The second answer would probably score 0. Although the idea of the same element and different number of neutrons is mentioned, the student has not mentioned different a ...
Chapter 3
... Mass Number and Isotopes • The stable oxygen isotopes provide another example. • 16O is the most abundant stable O isotope. • How many protons and neutrons are in 16O? 8 protons and 8 neutrons 17O is the least abundant stable O isotope. How many protons and neutrons are in 17O? 8 protons and 9 ...
... Mass Number and Isotopes • The stable oxygen isotopes provide another example. • 16O is the most abundant stable O isotope. • How many protons and neutrons are in 16O? 8 protons and 8 neutrons 17O is the least abundant stable O isotope. How many protons and neutrons are in 17O? 8 protons and 9 ...
Chapter 3
... Mass Number and Isotopes • The stable oxygen isotopes provide another example. • 16O is the most abundant stable O isotope. • How many protons and neutrons are in 16O? 8 protons and 8 neutrons 17O is the least abundant stable O isotope. How many protons and neutrons are in 17O? 8 protons and 9 ...
... Mass Number and Isotopes • The stable oxygen isotopes provide another example. • 16O is the most abundant stable O isotope. • How many protons and neutrons are in 16O? 8 protons and 8 neutrons 17O is the least abundant stable O isotope. How many protons and neutrons are in 17O? 8 protons and 9 ...
The Basics of Atomic Structure
... • An Isotope is an atom with the same number of protons but a different number of neutrons; therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. • An Ion is an element with a number of electrons that d ...
... • An Isotope is an atom with the same number of protons but a different number of neutrons; therefore, the mass number will be different for the same element. All atoms of an element are considered an isotope, some are more common than others. • An Ion is an element with a number of electrons that d ...
Atomic Structure
... b. Credited with the discovery of the neutron c. Credited with the discovery of the electron and the “plum pudding” model of the atom. d. Used the now famous “gold foil” experiment to prove the existence of the nucleus. He also showed most of an atom is empty space! e. Credited with the “planetary” ...
... b. Credited with the discovery of the neutron c. Credited with the discovery of the electron and the “plum pudding” model of the atom. d. Used the now famous “gold foil” experiment to prove the existence of the nucleus. He also showed most of an atom is empty space! e. Credited with the “planetary” ...
Atomic Structure Worksheet
... 4. Atoms of the same element that differ in their number of neutrons in the nucleus are called isotopes. 5. The total number of nucleons (particles in the nucleus) in the atom make up the mass number. 6. A neutral nuclear particle having a mass of about 1 AMU is called the neutron. 7. The proton is ...
... 4. Atoms of the same element that differ in their number of neutrons in the nucleus are called isotopes. 5. The total number of nucleons (particles in the nucleus) in the atom make up the mass number. 6. A neutral nuclear particle having a mass of about 1 AMU is called the neutron. 7. The proton is ...
Periodic Table
... protons) will appear at the __________ • The mass number (number of protons plus neutrons will appear at the __________ • The element symbol will appear to the __________ • The different number of neutrons has NO bearing on chemical reactivity ...
... protons) will appear at the __________ • The mass number (number of protons plus neutrons will appear at the __________ • The element symbol will appear to the __________ • The different number of neutrons has NO bearing on chemical reactivity ...
Honors Review Unit 2 answers
... Used an “oil drop” experiment to discover the charge and mass of an electron. ___Millikan__ Proposed the “Plum Pudding” model of the atom. _____Thomson________ Used the word “atomos” to describe matter. _____Democritos________ Called the “Father of Modern Chemistry”. ___Lavoisier_________ Discovered ...
... Used an “oil drop” experiment to discover the charge and mass of an electron. ___Millikan__ Proposed the “Plum Pudding” model of the atom. _____Thomson________ Used the word “atomos” to describe matter. _____Democritos________ Called the “Father of Modern Chemistry”. ___Lavoisier_________ Discovered ...
atomic number - Thomas C. Cario Middle School
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
... The periodic table is a chart containing information about the atoms that make up all matter. An element is a substance made up of only one type of atom. The atomic number of an atom is equal to the number of protons in its nucleus. The number of electrons surrounding the nucleus of an atom is equal ...
Atomic Structure
... • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
... • If an atom were the size of a football stadium, the nucleus would be about the size of a marble. ...
SCI 3101 Test IV MULTIPLE CHOICE. 1) The sky is blue because air
... 14) If an atom has 43 electrons, 56 neutrons, and 43 protons, what is its approximate atomic mass? What is the name of this element? A) Atomic mass 142 amu; Einsteinium (Es) B) Atomic mass, 99 amu; Radon (Ra) C) Atomic mass, 137 amu; Barium (Ba) D) Atomic mass, 99 amu; Technetium (Tc) 15) Why are t ...
... 14) If an atom has 43 electrons, 56 neutrons, and 43 protons, what is its approximate atomic mass? What is the name of this element? A) Atomic mass 142 amu; Einsteinium (Es) B) Atomic mass, 99 amu; Radon (Ra) C) Atomic mass, 137 amu; Barium (Ba) D) Atomic mass, 99 amu; Technetium (Tc) 15) Why are t ...
Chapter 3
... 2. All atoms of a given element have identical properties that differ from those of other elements. 3. Atoms cannot be created, destroyed, or transformed into atoms of another element. 4. Compounds are formed when atoms of different elements combine with one another in small wholenumber ratios. 5. T ...
... 2. All atoms of a given element have identical properties that differ from those of other elements. 3. Atoms cannot be created, destroyed, or transformed into atoms of another element. 4. Compounds are formed when atoms of different elements combine with one another in small wholenumber ratios. 5. T ...
Atoms and Molecules
... • Elements are determined by the number of protons in the nucleus of the atom ...
... • Elements are determined by the number of protons in the nucleus of the atom ...
Isotope Practice Worksheet
... Although the amount of heavy water molecules in nature is very small (1 part in 3200), it has been concentrated for use in the development of nuclear weapons. Also, when concentrated, it can be poisonous to plants and animals. ...
... Although the amount of heavy water molecules in nature is very small (1 part in 3200), it has been concentrated for use in the development of nuclear weapons. Also, when concentrated, it can be poisonous to plants and animals. ...
Kentucky newspapers 1949 look at the city, part 5
... beta rays. Geigers aren’t so good on detecting alpha rays, but other devices take care of that. Now, how is a radioisotope made? Certain amounts of basic elements are inserted into the pile. There, they are bombarded with neutrons from the splitting U-235 atom. These neutron “bullets” either knock p ...
... beta rays. Geigers aren’t so good on detecting alpha rays, but other devices take care of that. Now, how is a radioisotope made? Certain amounts of basic elements are inserted into the pile. There, they are bombarded with neutrons from the splitting U-235 atom. These neutron “bullets” either knock p ...
Unit 3 Note Outline
... History of the Atom Democritis - Ancient Greek Philosopher used the word Two discoveries led to the rebirth of the idea of the atom 1. Lavoisier 2. Proust (1799) This led to: ...
... History of the Atom Democritis - Ancient Greek Philosopher used the word Two discoveries led to the rebirth of the idea of the atom 1. Lavoisier 2. Proust (1799) This led to: ...
04 Atoms_ molecules _ ions
... •Left three quarters of the chart •Lose electrons •Become positive ...
... •Left three quarters of the chart •Lose electrons •Become positive ...
Einsteinium

Einsteinium is a synthetic element with symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein. Its most common isotope einsteinium-253 (half life 20.47 days) is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but at much smaller amounts, by bombarding heavy actinide elements with light ions. Owing to the small amounts of produced einsteinium and the short half-life of its most easily produced isotope, there are currently almost no practical applications for it outside of basic scientific research. In particular, einsteinium was used to synthesize, for the first time, 17 atoms of the new element mendelevium in 1955.Einsteinium is a soft, silvery, paramagnetic metal. Its chemistry is typical of the late actinides, with a preponderance of the +3 oxidation state; the +2 oxidation state is also accessible, especially in solids. The high radioactivity of einsteinium-253 produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watts per gram. Difficulty in studying its properties is due to einsteinium-253's conversion to berkelium and then californium at a rate of about 3% per day. The isotope of einsteinium with the longest half life, einsteinium-252 (half life 471.7 days) would be more suitable for investigation of physical properties, but it has proven far more difficult to produce and is available only in minute quantities, and not in bulk. Einsteinium is the element with the highest atomic number which has been observed in macroscopic quantities in its pure form, and this was the common short-lived isotope einsteinium-253.Like all synthetic transuranic elements, isotopes of einsteinium are very radioactive and are considered highly dangerous to health on ingestion.