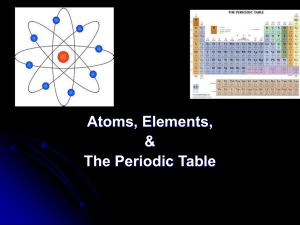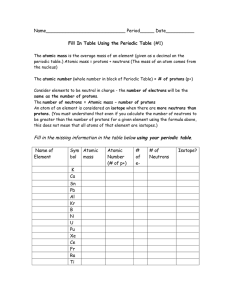
Periodic Table Fill in Table 1
... same as the number of protons. The number of neutrons = Atomic mass – number of protons An atom of an element is considered an isotope when there are more neutrons than protons. (You must understand that even if you calculate the number of neutrons to be greater than the number of protons for a give ...
... same as the number of protons. The number of neutrons = Atomic mass – number of protons An atom of an element is considered an isotope when there are more neutrons than protons. (You must understand that even if you calculate the number of neutrons to be greater than the number of protons for a give ...
10B Atoms and Isotopes
... mass number = number of protons + number of neutrons Atoms of the same element always have the same number of protons, but can have different numbers of neutrons. These different forms of the same element are called isotopes. Example: Sometimes the mass number for an element is included in its symbo ...
... mass number = number of protons + number of neutrons Atoms of the same element always have the same number of protons, but can have different numbers of neutrons. These different forms of the same element are called isotopes. Example: Sometimes the mass number for an element is included in its symbo ...
Atom
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
... 1904: J.J.Thompson discovered electrons & proposed the “plum pudding model” 1911: Earnest Rutherford discovered the nucleus. 1913: Neils Bohr proposed that electrons orbit with electrostic forces rather than gravity. the “planetary model” 1926: Erwin Schrodinger analyzed electron orbits from a geome ...
Ch4StudyGuide
... Why do most atoms have no charge even though they are made up of positively charged protons and negatively charged electrons? ...
... Why do most atoms have no charge even though they are made up of positively charged protons and negatively charged electrons? ...
14_1_atoms and isotopes FPS3
... element. The atomic mass however, increases by amounts greater than one. This difference is due to the neutrons in the nucleus. The value of the atomic mass reflects the abundance of the stable isotopes for an element that exist in the universe. Since silver has an atomic mass of 107.87, this means ...
... element. The atomic mass however, increases by amounts greater than one. This difference is due to the neutrons in the nucleus. The value of the atomic mass reflects the abundance of the stable isotopes for an element that exist in the universe. Since silver has an atomic mass of 107.87, this means ...
Homework 1B1 - 3 - Uddingston Grammar School
... (a) Complete the diagram to show how the electrons are arranged. You may wish to use the data booklet to help you. ...
... (a) Complete the diagram to show how the electrons are arranged. You may wish to use the data booklet to help you. ...
12.1 Atoms and Isotopes
... Sometimes the mass number for an element is included in its symbol. When the symbol is written in this way, we call it isotope notation. The isotope notation for carbon-12 is shown to the right. How many neutrons does an atom of carbon-12 have? To find out, simply take the mass number and subtract t ...
... Sometimes the mass number for an element is included in its symbol. When the symbol is written in this way, we call it isotope notation. The isotope notation for carbon-12 is shown to the right. How many neutrons does an atom of carbon-12 have? To find out, simply take the mass number and subtract t ...
Document
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
Abstract
... changing isotope ratios, because heavier isotopes are more difficult to move than lighter ones. Such isotope changes are called mass-dependent fractionation. The large isotope fractionation takes place between two isotopes with a large mass difference. In the case of oxygen, the fractionation in (18 ...
... changing isotope ratios, because heavier isotopes are more difficult to move than lighter ones. Such isotope changes are called mass-dependent fractionation. The large isotope fractionation takes place between two isotopes with a large mass difference. In the case of oxygen, the fractionation in (18 ...
PP 04 Atoms_ molecules_ ions
... Atomic Theory: Elements composed of atoms. Atoms can’t be changed. Compounds comtain multiples of atoms. John Dalton The Law of Conservation of Mass: In ordinary chemical reactions, matter can be neither created nor destroyed. The Law of Constant Composition: Compounds always have the same proportio ...
... Atomic Theory: Elements composed of atoms. Atoms can’t be changed. Compounds comtain multiples of atoms. John Dalton The Law of Conservation of Mass: In ordinary chemical reactions, matter can be neither created nor destroyed. The Law of Constant Composition: Compounds always have the same proportio ...
Ch. 2 note packet
... Metallurgy - extraction of metals from ore Robert Boyle - (1661 - Skeptical Chymist) - first quantitative experiments; current concept of “element” Georg Stahl – suggested “phlogiston” flowed out of burning material Joseph Priestley - (1733-1804) “discovered” oxygen (not phlogiston) 2.2 - 2.3 Fundam ...
... Metallurgy - extraction of metals from ore Robert Boyle - (1661 - Skeptical Chymist) - first quantitative experiments; current concept of “element” Georg Stahl – suggested “phlogiston” flowed out of burning material Joseph Priestley - (1733-1804) “discovered” oxygen (not phlogiston) 2.2 - 2.3 Fundam ...
Name Test Review Chemistry Unit 2: The Atom 1. Fill in the blank
... 72.2% and the abundance of X is 27.8%, what is the average atomic mass of X? What is element X? 5. Silicon has three naturally occurring isotopes. What is the average atomic mass of silicon? ...
... 72.2% and the abundance of X is 27.8%, what is the average atomic mass of X? What is element X? 5. Silicon has three naturally occurring isotopes. What is the average atomic mass of silicon? ...
Atomic Mass
... Atomic masses can be different for atoms of the same element if they have different numbers of neutrons Atoms with different masses are called Isotopes or Nuclides ...
... Atomic masses can be different for atoms of the same element if they have different numbers of neutrons Atoms with different masses are called Isotopes or Nuclides ...
Name Test Review Chapters 4 and 25 Honors Chemistry 1. Fill in
... 17. During beta decay, describe what happens to the atomic number of the element. 18. What is the most penetrating form of radioactive particles? What can stop this form of radioactivity? ...
... 17. During beta decay, describe what happens to the atomic number of the element. 18. What is the most penetrating form of radioactive particles? What can stop this form of radioactivity? ...
Isotopes, Ions Worksheet
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
... b) Do different isotopes have different half-lifes (t ½ )? YES Different isotopes have a different neutron number which results in different half-life 21. List THREE Nuclear Applications 1. _______________________ 2. ______________________ 3. _______________________ 22. Why is it important to know t ...
PP - myndrs.com
... Bohr’s Model of Electrons: • Suggested that electrons move around the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: ...
... Bohr’s Model of Electrons: • Suggested that electrons move around the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: ...
Structure of an Atom structure_of_atom
... Bohr’s Model of Electrons: • Suggested that electrons move around the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: ...
... Bohr’s Model of Electrons: • Suggested that electrons move around the nucleus of an atom in orbits or shells. • Each orbit is a certain distance from the nucleus and contains a definite number of electrons. • The orbits are filled in a routine way: ...
Extension 18.2: Isotopes
... Naturally-occurring hydrogen, 99.985% of it, is 1 H, and the rest, 0.015%, is deuterium, ...
... Naturally-occurring hydrogen, 99.985% of it, is 1 H, and the rest, 0.015%, is deuterium, ...
Atomic Structure AKS Correlation Use the modern atomic theory to
... Penetrating power /Shielding/stopped by? ...
... Penetrating power /Shielding/stopped by? ...
Lesson 13 - Highline Public Schools
... weighted average of the masses of the isotopes in a sample of the element. The most common isotope of an element, frequently has a mass that is close to the average atomic mass given in the periodic table. ...
... weighted average of the masses of the isotopes in a sample of the element. The most common isotope of an element, frequently has a mass that is close to the average atomic mass given in the periodic table. ...
Structure of the Atom
... • The mass number is the total number of protons and neutrons in the nucleus. • Atoms can have different numbers of neutrons. • Atoms that have different numbers of neutrons are called isotopes. ...
... • The mass number is the total number of protons and neutrons in the nucleus. • Atoms can have different numbers of neutrons. • Atoms that have different numbers of neutrons are called isotopes. ...
Atomic Structure
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
... •Even though isotopes have different amounts of neutrons they are still chemically alike since they have the same number of protons and electrons. •To find the most common isotope round the atomic mass to nearest whole number. -Ex: Carbon-12 is the most common isotope of carbon Which isotope is the ...
Isotopes
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
... Isotopes are atoms of the same element having different masses, due to varying numbers of neutrons. ...
Chapter 1 Learning Objective Summary
... changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioactive decay or bombardment with another particle. Many isotopes are unstable, and undergo spontaneous radioactive d ...
... changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another can be accomplished via radioactive decay or bombardment with another particle. Many isotopes are unstable, and undergo spontaneous radioactive d ...
Einsteinium

Einsteinium is a synthetic element with symbol Es and atomic number 99. It is the seventh transuranic element, and an actinide.Einsteinium was discovered as a component of the debris of the first hydrogen bomb explosion in 1952, and named after Albert Einstein. Its most common isotope einsteinium-253 (half life 20.47 days) is produced artificially from decay of californium-253 in a few dedicated high-power nuclear reactors with a total yield on the order of one milligram per year. The reactor synthesis is followed by a complex process of separating einsteinium-253 from other actinides and products of their decay. Other isotopes are synthesized in various laboratories, but at much smaller amounts, by bombarding heavy actinide elements with light ions. Owing to the small amounts of produced einsteinium and the short half-life of its most easily produced isotope, there are currently almost no practical applications for it outside of basic scientific research. In particular, einsteinium was used to synthesize, for the first time, 17 atoms of the new element mendelevium in 1955.Einsteinium is a soft, silvery, paramagnetic metal. Its chemistry is typical of the late actinides, with a preponderance of the +3 oxidation state; the +2 oxidation state is also accessible, especially in solids. The high radioactivity of einsteinium-253 produces a visible glow and rapidly damages its crystalline metal lattice, with released heat of about 1000 watts per gram. Difficulty in studying its properties is due to einsteinium-253's conversion to berkelium and then californium at a rate of about 3% per day. The isotope of einsteinium with the longest half life, einsteinium-252 (half life 471.7 days) would be more suitable for investigation of physical properties, but it has proven far more difficult to produce and is available only in minute quantities, and not in bulk. Einsteinium is the element with the highest atomic number which has been observed in macroscopic quantities in its pure form, and this was the common short-lived isotope einsteinium-253.Like all synthetic transuranic elements, isotopes of einsteinium are very radioactive and are considered highly dangerous to health on ingestion.