Atoms, Isotopes, and Ions
... Since silver has an atomic mass of 107.87, this means that most of the stable isotopes that exist have a mass number of 108. In other words, the most common silver isotope is “silver-108.” To figure out the most common isotope for an element, round the atomic mass to the nearest whole number. 3. Loo ...
... Since silver has an atomic mass of 107.87, this means that most of the stable isotopes that exist have a mass number of 108. In other words, the most common silver isotope is “silver-108.” To figure out the most common isotope for an element, round the atomic mass to the nearest whole number. 3. Loo ...
Abstract
... and 18O with 8, 9, and 10 neutrons, respectively, and are called oxygen isotopes. Isotopes differ in atomic weight but are similar in behavior during chemical reactions because they have the same number of electrons. Therefore, isotope ratios such as 18O/16O change little during chemical reactions. ...
... and 18O with 8, 9, and 10 neutrons, respectively, and are called oxygen isotopes. Isotopes differ in atomic weight but are similar in behavior during chemical reactions because they have the same number of electrons. Therefore, isotope ratios such as 18O/16O change little during chemical reactions. ...
ppt
... Most of the mass of an atom is in the small, dense nucleus. ● The radius of an atom is about 100,000 times larger than the radius of the nucleus. ● Electrons are located around the nucleus in orbitals. ● Orbitals – not distinct like planetary orbits, but 3-D regions where electrons can probably be ...
... Most of the mass of an atom is in the small, dense nucleus. ● The radius of an atom is about 100,000 times larger than the radius of the nucleus. ● Electrons are located around the nucleus in orbitals. ● Orbitals – not distinct like planetary orbits, but 3-D regions where electrons can probably be ...
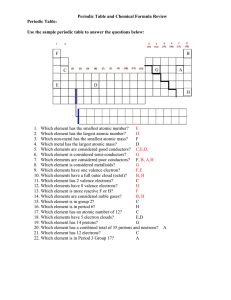
Periodic Table Review Key
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
Document
... How do isotopes of the same element differ? How are they the same? What are the two ways to write isotopes? Write both ways for boron (B) atomic number 5 and mass 11 ...
... How do isotopes of the same element differ? How are they the same? What are the two ways to write isotopes? Write both ways for boron (B) atomic number 5 and mass 11 ...
File - Norris Science
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
... the tiny alpha particles would pass through the gold atoms and fly straight into the screen. ...
Atomic Structure
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
... Different atoms have different ____________ and ____________ The differing properties of matter are due to the size, shape, and movement of ____________ Changes in matter result from changes in the ____________ of atoms and not the atoms themselves ...
Gr 10 Review sheet chemistry
... 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
... 2. Formation of a ________________ 3. Formation of _____________ 4. Release or absorption of_____________ ...
Keypoints of Basic Atomic Structure
... Atomic Number Atomic Radius Electrons Element Isotope Neutrons Periodic Table Protons Subatomic Particles Concepts 1. Be able to describe how protons, neutrons and electrons are arranged in an atom. 2. Be able to list the charges on the subatomic particles that make up and atom, and giv ...
... Atomic Number Atomic Radius Electrons Element Isotope Neutrons Periodic Table Protons Subatomic Particles Concepts 1. Be able to describe how protons, neutrons and electrons are arranged in an atom. 2. Be able to list the charges on the subatomic particles that make up and atom, and giv ...
ISOTOPES 3 SUBATOMIC PARTICLES Proton Located inside the
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
... Located outside of the nucleus in an “electron cloud” Involved in chemical bonding Negative charge Equal to the # of protons in a neutral atom How many electrons does Potassium have? How many electrons does Nitrogen have? o Neutron Located inside the nucleus of an atom No charge # ...
Atoms: The Building Blocks of Matter
... Atoms can lose or gain electrons when bonding to make ionic compounds We keep track of the number of electrons that can be lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus ...
... Atoms can lose or gain electrons when bonding to make ionic compounds We keep track of the number of electrons that can be lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus ...
Subatomic Heavyweights
... Atoms of the same element will ALWAYS have the same number of protons • Atomic weight: the weighted average atomic mass of the naturally occurring isotopes (the # on the periodic table) ...
... Atoms of the same element will ALWAYS have the same number of protons • Atomic weight: the weighted average atomic mass of the naturally occurring isotopes (the # on the periodic table) ...
1. Of the three major categories of elements (metals, non
... They are called groups or families. 12. What are the horizontal rows on the periodic table called? They are called periods. 13. Explain the relationship between elements in the same group. They have similar chemical and physical properties because each one has the same number of valence electrons. ...
... They are called groups or families. 12. What are the horizontal rows on the periodic table called? They are called periods. 13. Explain the relationship between elements in the same group. They have similar chemical and physical properties because each one has the same number of valence electrons. ...
Chapter 3 Atoms and Elements
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. According to the mathematics each s, p, d level can accommodate 2 electrons. There is one s level for each shelf, three equivalent ...
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. According to the mathematics each s, p, d level can accommodate 2 electrons. There is one s level for each shelf, three equivalent ...
22-Introduction to Radioactivity
... In this activity, you will first review some of the important ideas about atoms. Then you will begin to learn about two important ways in which radioactivity can occur. This prepares you for conducting an experiment that models what happens during radioactive decay. Reviewing Some Ideas about Atoms ...
... In this activity, you will first review some of the important ideas about atoms. Then you will begin to learn about two important ways in which radioactivity can occur. This prepares you for conducting an experiment that models what happens during radioactive decay. Reviewing Some Ideas about Atoms ...
Basic structure of atoms
... • Electrons move very rapidly in complicated paths called orbitals. • Because of this motion, they appear to form a cloud. – Negative charge -1 – Mass: 9.1 x10-28 grams – Symbols include e-, -1e0 ...
... • Electrons move very rapidly in complicated paths called orbitals. • Because of this motion, they appear to form a cloud. – Negative charge -1 – Mass: 9.1 x10-28 grams – Symbols include e-, -1e0 ...
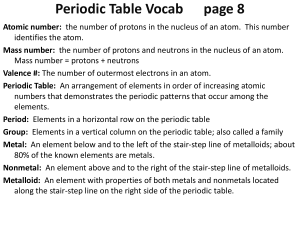
Periodic Table Vocab page 7
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
... Mass number: the number of protons and neutrons in the nucleus of an atom. Mass number = protons + neutrons Valence #: The number of outermost electrons in an atom. Periodic Table: An arrangement of elements in order of increasing atomic numbers that demonstrates the periodic patterns that occur amo ...
ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 31. What are the three types of radioactive decay you learned about? Write the symbol for each (include all relevant numbers). ...
... 31. What are the three types of radioactive decay you learned about? Write the symbol for each (include all relevant numbers). ...
The Periodic Table
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
... Mass number is the count of nucleons in an isotope and atomic mass is the measure of the average mass of an atom including the relative abundance of its element’s isotopes. ...
Vocabulary and Section Summary
... Name ______________________________ Class___________________Date__________________ ...
... Name ______________________________ Class___________________Date__________________ ...
Lecture 3
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. ...
... Also on closer inspection of the different n levels, additional fine structure is observed within each n level and these are assigned different letters of the alphabet. ...
Lawrencium

Lawrencium is a synthetic chemical element with chemical symbol Lr (formerly Lw) and atomic number 103. It is named in honor of Ernest Lawrence, inventor of the cyclotron, a device that was used to discover many artificial radioactive elements. A radioactive metal, lawrencium is the eleventh transuranic element and is also the final member of the actinide series. Like all elements with atomic number over 100, lawrencium can only be produced in particle accelerators by bombarding lighter elements with charged particles. Twelve isotopes of lawrencium are currently known; the most stable is 266Lr with a half-life of 11 hours, but the shorter-lived 260Lr (half-life 2.7 minutes) is most commonly used in chemistry because it can be produced on a larger scale.Chemistry experiments have confirmed that lawrencium indeed behaves as a heavier homolog to lutetium in the periodic table, and is a trivalent element. It thus could also be classified as the first of the 7th-period transition metals: however, its electron configuration is anomalous for its position in the periodic table, having an s2p configuration instead of the s2d configuration of its homolog lutetium. This means that lawrencium may be less volatile than expected for its position in the periodic table and have a volatility comparable to that of lead.In the 1950s, 1960s, and 1970s, many claims of the synthesis of lawrencium of varying quality were made from laboratories in the Soviet Union and the United States. The priority of the discovery and therefore the naming of the element was disputed between Soviet and American scientists, and while the International Union of Pure and Applied Chemistry (IUPAC) established lawrencium as the official name for the element and gave the American team credit for the discovery, this was reevaluated in 1997, giving both teams shared credit for the discovery but not changing the element's name.