Lecture I
... the very small intermolecular attractions of this atom. • Tc of the noble gas elements increases with atomic number. • Hydrogen gas cannot be liquified above 33 K; this poses a major difficulty in the use of hydrogen as an automotive fuel; storage as a high-pressure gas requires heavy steel containe ...
... the very small intermolecular attractions of this atom. • Tc of the noble gas elements increases with atomic number. • Hydrogen gas cannot be liquified above 33 K; this poses a major difficulty in the use of hydrogen as an automotive fuel; storage as a high-pressure gas requires heavy steel containe ...
The Chemical Earth (8.2.3)
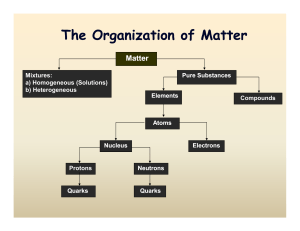
... difference between solids, liquids and gases. 2. In terms of size and bonding, what is the difference between argon, oxygen, and helium. 3. Draw Lewis electron dot diagrams for: a. ...
... difference between solids, liquids and gases. 2. In terms of size and bonding, what is the difference between argon, oxygen, and helium. 3. Draw Lewis electron dot diagrams for: a. ...
ChE 215, Physical Chemistry
... Class Hours : Weekdays (9:30 - 11:00) Lab Hours : Saturday (2:00 - 5:00 p.m.) Class Room : Bldg. 6 Kh., Room # 305 Course Objective: The objective of this course is to introduce the basic topics in Kinetics, Thermodynamics and Statistical Mechanics. In fact this course present collection of distinct ...
... Class Hours : Weekdays (9:30 - 11:00) Lab Hours : Saturday (2:00 - 5:00 p.m.) Class Room : Bldg. 6 Kh., Room # 305 Course Objective: The objective of this course is to introduce the basic topics in Kinetics, Thermodynamics and Statistical Mechanics. In fact this course present collection of distinct ...
6 Departure from thermal equilibrium
... is the Planck mass. If the scattering process only involves massless particles, including in intermediate states, then by dimensional grounds it must go as Γ ∼ α2 T , where α = g 2 /4π and g is a typical coupling constant in the process. Thus in the very early universe, when T /Mp & α2 , this scatte ...
... is the Planck mass. If the scattering process only involves massless particles, including in intermediate states, then by dimensional grounds it must go as Γ ∼ α2 T , where α = g 2 /4π and g is a typical coupling constant in the process. Thus in the very early universe, when T /Mp & α2 , this scatte ...