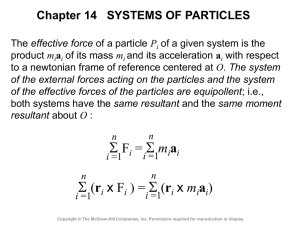
Lecture 10
... August 24, 1888), was a German physicist and mathematician, and was one of thefounders of thermodynamics. His most important paper, on the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. He ...
... August 24, 1888), was a German physicist and mathematician, and was one of thefounders of thermodynamics. His most important paper, on the mechanical theory of heat, published in 1850, first stated the basic ideas of the second law of thermodynamics. In 1865 he introduced the concept of entropy. He ...
Estimating the entropy of a signal with applications
... detecting law changes in signals. As an illustration, we consider a signal x (n) that consists in 400 samples generated according to a mixture of two Gaussian distributions, followed by 400 uniformly distributed samples. First law has entropy H1 = 1:8 whereas the second has entropy H2 = 1:18: Figure ...
... detecting law changes in signals. As an illustration, we consider a signal x (n) that consists in 400 samples generated according to a mixture of two Gaussian distributions, followed by 400 uniformly distributed samples. First law has entropy H1 = 1:8 whereas the second has entropy H2 = 1:18: Figure ...
Mechanics
... curves in G-space. The curve consists of all points at the same energy. A system whose phase trajectory covers all points at an energy is ergodic. Energy defines all states of the system ...
... curves in G-space. The curve consists of all points at the same energy. A system whose phase trajectory covers all points at an energy is ergodic. Energy defines all states of the system ...
Chapter 1 Introduction and Definition of Terms
... If body A and B are each in thermal equilibrium with a third body C, they are also in thermal equilibrium with each other. TA = TC and TB = TC => TA = TB The aim of classical thermodynamics is to establish the relationships which exist between equilibrium state of a given system and the influences w ...
... If body A and B are each in thermal equilibrium with a third body C, they are also in thermal equilibrium with each other. TA = TC and TB = TC => TA = TB The aim of classical thermodynamics is to establish the relationships which exist between equilibrium state of a given system and the influences w ...
Ch 10 equations - mvhs
... Gases Equations– AP Chemistry: Remember for STP problems, use 1mol/22.4 L and Non-STP problems, use ideal gas law PV=nRT, S.T.P.= 0°C and 1 atm, R.T.P.= 25° C and 1 atm Equation ...
... Gases Equations– AP Chemistry: Remember for STP problems, use 1mol/22.4 L and Non-STP problems, use ideal gas law PV=nRT, S.T.P.= 0°C and 1 atm, R.T.P.= 25° C and 1 atm Equation ...
10CH301 - Karunya University
... system – Zinc-magnesium alloy system – Copper-nickel alloy system – sodium-sulphate system – Ironcarbon alloy system – Three component system – Brief description about two salts and water system – Allotropy – Heat treatment. Unit IV Statistical Thermodynamics – I: Concepts of probability and Maxwell ...
... system – Zinc-magnesium alloy system – Copper-nickel alloy system – sodium-sulphate system – Ironcarbon alloy system – Three component system – Brief description about two salts and water system – Allotropy – Heat treatment. Unit IV Statistical Thermodynamics – I: Concepts of probability and Maxwell ...
Zumdahl Chapter
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...
... First Year Chemistry Podcast DVD Featuring Jonathan Bergmann and Aaron Sams from Peak Educational Consulting LLC All Rights Reserved © This is an interactive page that allows you to get to all of the content on this DVD. Click to each unit packet or podcast. The podcasts require Quicktime and the pa ...